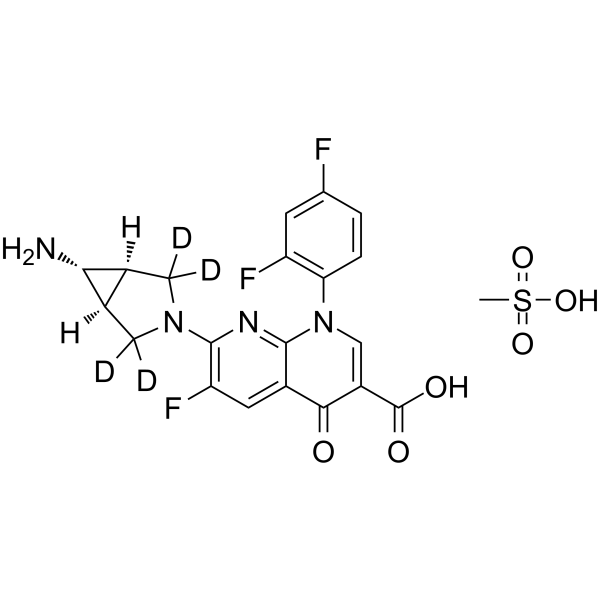
1346601-60-2
| Name | Trovafloxacin-d4 mesylate |
|---|
| Description | Trovafloxacin-d4 mesylate is the deuterium labeled Trovafloxacin mesylate. Trovafloxacin mesylate is a broad-spectrum quinolone antibiotic with potent activity against Gram-positive, Gram-negative and anaerobic organisms. Trovafloxacin mesylate blocks the DNA gyrase and topoisomerase IV activity. Trovafloxacin mesylate is also a potent, selective and orally active pannexin 1 channel (PANX1) inhibitor with an IC50 of 4 μM for PANX1 inward current. Trovafloxacin mesylate does not inhibit connexin 43 gap junction or PANX2. Trovafloxacin mesylate leads to dysregulated fragmentation of apoptotic cells by inhibiting PANX1[1][2][3]. |
|---|---|
| Related Catalog | |
| In Vitro | Stable heavy isotopes of hydrogen, carbon, and other elements have been incorporated into drug molecules, largely as tracers for quantitation during the drug development process. Deuteration has gained attention because of its potential to affect the pharmacokinetic and metabolic profiles of drugs[1]. |
| References |
| Molecular Formula | C21H15D4F3N4O6S |
|---|---|
| Molecular Weight | 516.48 |
| Hazard Codes | Xi |
|---|