28755-03-5
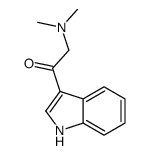
| Name | 2-chloro-1-(1H-indol-3-yl)ethanone |
|---|---|
| Synonyms |
BB_SC-0127
2-chloro-1-indol-3-ylethan-1-one indol-3-yl chloromethyl ketone 3CAI |
| Description | 3CAI is a potent and specific AKT1 and AKT2 inhibitor. |
|---|---|
| Related Catalog | |
| Target |
Akt1 Akt2 |
| In Vitro | 3CAI is a potential inhibitor of AKT. Based on these screening data, the effect of 3CAI on the kinase activities of AKT1, MEK1, JNK1, ERK1 and TOPK is tested using in vitro kinase assays. The results show that 3CAI (1 μM) suppresses only AKT1 kinase activity and the other kinases tested are not affected by 3CAI. 3CAI is a much more potent AKT1 inhibitor than PI3K (60% inhibition at 1 vs 10 μM, respectively). 3CAI substantially suppresses AKT1 activity as well as AKT2 activity in a dose dependent manner. 3CAI inhibits down-stream targets of AKT and induces apoptosis. AKT-mediated phosphorlyation site of mTOR (Ser2448) and GSK3β (Ser9) are substantially decreased by 3CAI in a time-dependent manner. Furthermore, pro-apoptotic marker proteins p53 and p21 are also upregulated by 3CAI after 12 or 24 h of treatment. HCT116 and HT29 colon cancer cells are seeded on 6 cm dishes in 1% FBS/McCoy's 5A (HCT116) with 3CAI (4 μM), I3C or the AKT inhibitor and then incubated for 4 days. Results show that the number of apoptotic cells is significantly increased by 3CAI in HCT116 and HT29 colon cancer cells compared with untreated control cells[1]. |
| In Vivo | To examine the antitumor activity of 3CAI in vivo, HCT116 cancer cells are injected into the right flank of individual athymic nude mice. Mice are orally administered 3CAI at 20 or 30 mg/kg, I3C at 100 mg/kg, or vehicle 5 times a week for 21 days. Treatment of mice with 30 mg/kg of 3CAI significantly suppresses HCT116 tumor growth by 50% relative to the vehicle-treated group (p<0.05). Remarkably, mice seem to tolerate treatment with these doses of 3CAI without overt signs of toxicity or significant loss of body weight compared with vehicle-treated group. Expression of these AKT-target proteins is strongly suppressed by 30 mg/kg of 3CAI in tumor tissues[1]. |
| Kinase Assay | The kinase assay is performed. Briefly, the reaction is carried out in the presence of 10 μCi of [γ-32P]ATP with each compound (e.g., 3CAI, 0.5, 1, 2 and 4 μM) in 40 μL of reaction buffer containing 20 mM HEPES (pH 7.4), 10 mM MgCl2, 10 mM MnCl2, and 1 mM dithiothreitol. After incubation at room temperature for 30 min, the reaction is stopped by adding 10 μL protein loading buffer and the mixture is separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The relative amounts of incorporated radioactivity are assessed by autoradiography[1]. |
| Cell Assay | HCT116 or HCT29 colon cancer cells are plated into 60-mm culture dishes (1×105 cells/dish) and incubated for 1 day in medium containing 10% FBS. The culture medium is then replaced with a 1% serum medium and cultured for 4 days with 3CAI (4 μM), I3C or a commercial AKT inhibitor. The cells are collected by trypsinization and washed with phosphate buffered saline (PBS). The cells are resuspended in 200 μL of binding buffer. Annexin V staining is accomplished. The cells are observed under a fluorescence microscope using a dual filter set for FITC and propidium iodide and then analyzed by flow cytometry[1]. |
| Animal Admin | Mice[1] Athymic mice [Cr:NIH(S), NIH Swiss nude, 6-9 wk old] are divided into five groups: 1) untreated vehicle group (n=15); 2) 20 mg 3CAI/kg of body weight (n=15), 3) 30 mg 3CAI/kg body weight (n=15); 4) 100 mg I3C/kg of body weight (n=15); 5) no cells and 30 mg 3CAI/kg of body weight (n=15). HCT116 cells (3×106 cells/100 μL) are suspended in serum free McCoy's 5A medium and inoculated subcutaneously into the right flank of each mouse. 3CAI, I3C or vehicle is administered orally 5 times per week for 21 days. Tumor volume is calculated. Mice are monitored until tumors reach 1 cm3 total volume, at which time mice are euthanized and tumors are extracted. |
| References |
| Density | 1.337g/cm3 |
|---|---|
| Boiling Point | 379.1ºC at 760 mmHg |
| Molecular Formula | C10H8ClNO |
| Molecular Weight | 193.63000 |
| Flash Point | 183ºC |
| Exact Mass | 193.02900 |
| PSA | 32.86000 |
| LogP | 2.58940 |
| Vapour Pressure | 6.02E-06mmHg at 25°C |
| Index of Refraction | 1.659 |
| Storage condition | 2-8℃ |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302-H319 |
| Precautionary Statements | P305 + P351 + P338 |
| RIDADR | NONH for all modes of transport |
| HS Code | 2933990090 |
|
~93% 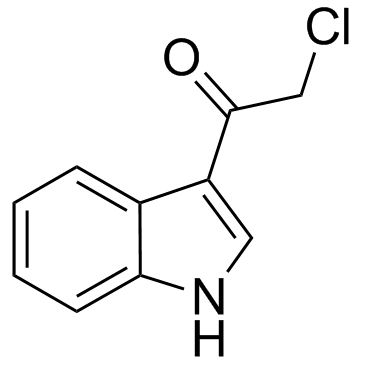
28755-03-5 |
| Literature: Abdel-Motaleb, Ramadan Maawad; Makhloof, Abdel-Moneim Abdel-Salam; Ibrahim, Hamada Mohamed; Elnagdi, Mohamed Hilmy Journal of Heterocyclic Chemistry, 2007 , vol. 44, # 1 p. 109 - 114 |
|
~% 
28755-03-5 |
| Literature: Preobrashenskaja et al. Chim.farm.Z., 1972 , vol. 6, # 1 p. 32,37;engl.Ausg.S.33,37 |
|
~% 
28755-03-5 |
| Literature: Majima; Kotake Chemische Berichte, 1922 , vol. 55, p. 3870 Chemische Berichte, 1930 , vol. 63, p. 2239 Full Text Show Details Sanna Gazzetta Chimica Italiana, 1929 , vol. 59, p. 845 |
|
~% 
28755-03-5 |
| Literature: Upjohn Co. Patent: GB869775 , 1959 ; |
|
~% 
28755-03-5 |
| Literature: Ames et al. Journal of the Chemical Society, 1956 , p. 1984,1988 |
|
~% 
28755-03-5 |
| Literature: Ames et al. Journal of the Chemical Society, 1956 , p. 1984,1988 |
| Precursor 6 | |
|---|---|
| DownStream 10 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |

![2-[2-(3,4-dimethoxyphenyl)ethylamino]-1-(1H-indol-3-yl)ethanone structure](https://image.chemsrc.com/caspic/324/113369-28-1.png)