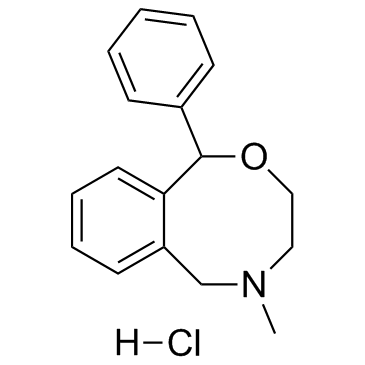
13669-70-0
| Name | 3-Methyl-7-phenyl-6-oxa-3-azabicyclo[6.4.0]dodeca-8,10,12-triene |
|---|---|
| Synonyms |
5-Methyl-1-phenyl-3,4,5,6-tetrahydro-1H-2,5-benzoxazocine
Sinalgico EINECS 237-148-2 5-methyl-1-phenyl-3,4,5,6-tetrahydro-1H-2,5-benzoxazocine hydrochloride 5-Methyl-1-phenyl-3,4,5,6-tetrahydro-1H-benzo[f][1,4]oxazocine Ajan 3,4,5,6-Tetrahydro-5-methyl-1-phenyl-1H-2,5-benzoxazocine Nefopam MFCD00056181 Acupan |
| Description | Nefopam (Fenazoxine) is an orally active, non-opioid and non-steroidal centrally acting analgesic agent. Nefopam blocks voltage-sensitive sodium channels (IC50=27 µM) and modulates glutamatergic transmission in rodents. Nefopam can be used in studies of neuropathic pain, anticonvulsant, as well as the prevention of postoperative shivering and hiccups[1][2][3]. |
|---|---|
| Related Catalog | |
| In Vitro | Nefopam (0.1-100 µM; 15 min) inhibits the uptake of 22Na in a concentration-dependent manner in SK-N-SH cells[1]. Cell Viability Assay[1] Cell Line: SK-N-SH cells Concentration: 0.1-100 µM Incubation Time: 15 min (preincubate) Result: Inhibited the uptake of 22Na with an IC50 value of 27 µM. |
| In Vivo | Nefopam (0-10 mg/kg; i.v.; single) protects mice against electroshock induced seizures[1].Nefopam (10, 30, 60 mg/kg; i.p.; single) shows a dose-dependent attenuation of mechanical allodynia and decreased neurokinin-1 receptor concentration in vincristine-induced peripheral neuropathy model[2]. Animal Model: Adult male NMRI mice (25-30 g; 6 to7-week-old; electroshock-induced seizures model)[1] Dosage: 0-10 mg/kg Administration: Intravenous injection; single Result: Produced dose-dependent protection against maximal electroshock seizures in mice with an ED50 of 3.8 (2.9-5.1) mg/kg. Animal Model: Adult male mice (10-week-old; 25-30 g; vincristine-induced peripheral neuropathy model)[2]. Dosage: 10, 30, 60 mg/kg Administration: Intraperitoneal injection; single Result: Significantly decreased the percentage of NK1 receptors in the spinal cord at dosage of 60 mg/kg. Showed a sustained increase in paw withdrawal threshold against mechanical stimuli. |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 369.5±37.0 °C at 760 mmHg |
| Molecular Formula | C17H19NO |
| Molecular Weight | 253.339 |
| Flash Point | 109.0±28.8 °C |
| Exact Mass | 253.146667 |
| PSA | 12.47000 |
| LogP | 3.44 |
| Vapour Pressure | 0.0±0.8 mmHg at 25°C |
| Index of Refraction | 1.564 |
| Storage condition | 2-8°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| HS Code | 2933990090 |
|---|
|
~98% 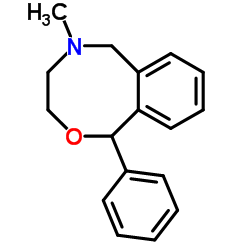
13669-70-0 |
| Literature: ARAKIS LTD. Patent: WO2005/56539 A2, 2005 ; Location in patent: Page/Page column 3 ; |
|
~% 
13669-70-0 |
| Literature: Australian Journal of Chemistry, , vol. 35, # 11 p. 2307 - 2314 |
|
~% 
13669-70-0 |
| Literature: Australian Journal of Chemistry, , vol. 35, # 11 p. 2307 - 2314 |
|
~% 
13669-70-0 |
| Literature: Australian Journal of Chemistry, , vol. 35, # 11 p. 2307 - 2314 |
| Precursor 3 | |
|---|---|
| DownStream 1 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |