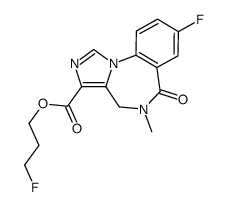
78755-81-4
| Name | flumazenil |
|---|---|
| Synonyms |
Romazicon
Lanexat Anexate Ethyl 8-fluoro-5-methyl-6-oxo-5,6-dihydro-4H-benzo[f]imidazo[1,5-a][1,4]diazepine-3-carboxylate Ro-15-1788 Ro 15-1788 YM-684 Ro 1722 Ethyl 8-fluoro-5-methyl-6-oxo-5,6-dihydro-4H-imidazo[1,5-a][1,4]benzodiazepine-3-carboxylate 4H-Imidazo[1,5-a][1,4]benzodiazepine-3-carboxylic acid, 8-fluoro-5,6-dihydro-5-methyl-6-oxo-, ethyl ester Mazicon Flumazenil FMZ Flumazepil 8-Fluoro-5,6-dihydro-5-methyl-6-oxo-4H-imidazo[1,5-a][1,4]benzodiazepine-3-carboxylic acid ethyl ester Ethyl-8-fluoro-5,6-dihydro-5-methyl-6-oxo-4H-imidazo[1,5-a][1,4]benzodiazepine-3-carboxylate 4H-Imidazo(1,5-a)(1,4)benzodiazepine-3-carboxylic acid, 8-fluoro-5,6-dihydro-5-methyl-6-oxo-, ethyl ester MFCD00242764 |
| Description | Flumazenil is a competitive GABAA receptor antagonist, used in the treatment of benzodiazepine overdoses. |
|---|---|
| Related Catalog | |
| In Vivo | Flumazenil interacts at the central benzodiazepine receptor to antagonize or reverse the behavioral, neurologic, and electrophysiologic effects of benzodiazepine agonists and inverse agonists. Flumazenil is of some benefit in hepatic encephalopathy, but until well-designed clinical trials are conducted, hepatic encephalopathy must be considered an investigational indication for flumazenil. Flumazenil has been shown to reverse sedation caused by intoxication with benzodiazepines alone or benzodiazepines in combination with other agents, but it should not be used when cyclic antidepressant intoxication is suspected[1]. Flumazenil (1 mg/kg) induces a strong anxiolytic effect in BALB/c mice tested in the elevated plus maze and light/dark test[2]. Flumazenil (10 mg/kg) effectively prevents the reduction produced by allopregnanolone in rats[3]. Flumazenil (5-20 mg/kg) antagonizes the anticonvulsant and adverse effects of diazepam but not GYKI 52466 in mice. Flumazenil slightly reduces the anticonvulsant activity of NBQX in the MES model but not in the PTZ test[4]. Flumazenil (3.0 mg/kg) blocks the changes withdrawal from chronic ethanol treatment, which leads to a decrease in open arm time and percent open arm entries[5]. |
| Animal Admin | Flumazenil is administered intraperitoneally in a volume of 10 mL/kg body weight 20 min before experimental testing. Two polyvinylchloride boxes (20×20×14 cm) covered with Plexiglas are connected by an opaque plastic tunnel (5×7×10 cm). One of these boxes is darkened. A light from a 100-W desk lamp 40 cm above the other box provided the only room illumination. This light level (300 lux) is chosen in order to avoid strain differences to be detected on time in the lit box (a measure of anxiety behaviour) in control animals. Indeed, in previous experiments, the BALB/c mice differ from C57BL/6 only in the high light condition. The subjects are individually tested in 5-min sessions between 1400 and 1700 hours. Mice are placed in the lit box to start the test session. The time spent in the lit box and the number of transitions from the dark box to the lit one are recorded after the first entry in the tunnel. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 528.0±50.0 °C at 760 mmHg |
| Melting Point | 201-203°C |
| Molecular Formula | C15H14FN3O3 |
| Molecular Weight | 303.288 |
| Flash Point | 273.1±30.1 °C |
| Exact Mass | 303.101929 |
| PSA | 64.43000 |
| LogP | 0.67 |
| Appearance | solid | white |
| Vapour Pressure | 0.0±1.4 mmHg at 25°C |
| Index of Refraction | 1.634 |
| Storage condition | 2-8°C |
| Water Solubility | 128 mg/L |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xi |
| Risk Phrases | R36/37/38:Irritating to eyes, respiratory system and skin . |
| Safety Phrases | S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice . S27:Take off immediately all contaminated clothing . S36/37/39:Wear suitable protective clothing, gloves and eye/face protection . |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 2 |
| RTECS | NI2922170 |
| HS Code | 2933990090 |
| Precursor 9 | |
|---|---|
| DownStream 2 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
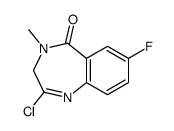

![8-amino-5,6-dihydro-5-methyl-6-oxo-4H-imidazol[1,5-a][1,4]benzodiazepine-3-carboxylic acid ethyl ester structure](https://image.chemsrc.com/caspic/482/658075-93-5.png)