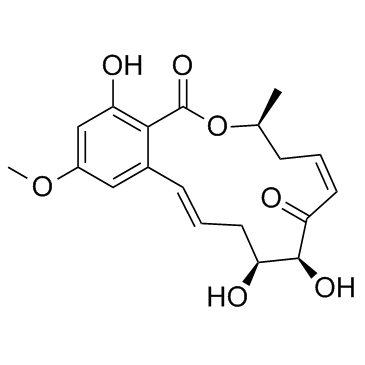
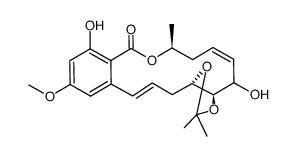
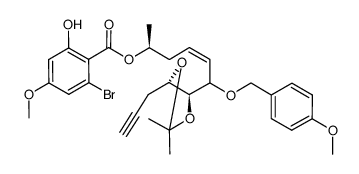
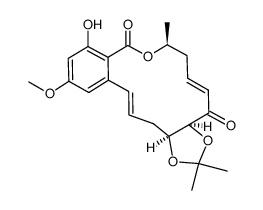
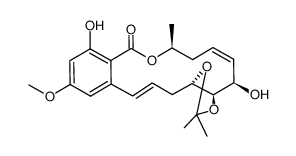
76958-67-3
| Name | hypothemycin |
|---|---|
| Synonyms |
(1aR,3S,4S,6Z,9S,15bR)-1a,8,9,15b-Tetrahydro-3,4,12-trihydroxy-9-methyl-14-methoxy-3H-oxireno[k][2]benzoxacyclotetradecin-5,11(2H,4H)-dione
MFCD08457932 Hypothemicin (1aR,3S,4S,6Z,9S,15bR)-1a,2,4,5,11,8,9,15b-Octahydro-3,4,12-trihydroxy-9-methyl-14-methoxy-3H-oxireno[k][2]benzoxacyclotetradecin-5,11-dione |
| Description | Hypothemycin, a fungal polyketide, is a multikinase inhibitor with Kis of 10/70 nM, 17/38 nM, 90 nM, 900 nM/1.5 μM, and 8.4/2.4 μM for VEGFR2/VEGFR1, MEK1/MEK2, FLT-3, PDGFRβ/PDGFRα, and ERK1/ERK2, respectively[1][2]. |
|---|---|
| Related Catalog | |
| Target |
VEGFR2:10 nM (Ki) VEGFR1:70 nM (Ki) MEK1:17 nM (Ki) MEK2:38 nM (Ki) FLT-3:90 nM (Ki) PDGFRα:1.5 μM (Ki) PDGFRβ:900 nM (Ki) ERK1:8.4 μM (Ki) ERK2:2.4 μM (Ki) |
| In Vitro | Hypothemycin inhibits eosinophilic EOL1 cell carrying mutations in FIP1L1-PDGFRα, acute myeloid leukemia MV-4-11 cell carrying mutations in FLT3-ITD, melanoma COLO829 cell carrying mutations in BRAF V600E, HUVEC cell carrying mutations in VEGFR, mastocytoma P815 cell carrying mutations in c-KIT D814Y, NSCLC A459 cell carrying mutations in KRAS, and ovarian SKOV-3 cell carrying mutations in HER2 with IC50s of 0.4 nM, 6 nM, 50 nM, 70 nM, 370 nM, 6.0 μM, and 7.0 μM, respectively[2]. |
| In Vivo | Hypothemycin (10 mg/kg) kills T. brucei in infected mice, completely curing the infection in one third of animals, although high doses of Hypothemycin (>10 mg/kg) leads to side effects[1]. Animal Model: Adult female Balb/c mice (weighing 18–22 g) infected with T. brucei[1] Dosage: 10 mg/kg Administration: Administered once daily via intraperitoneal injection for 7 days. Result: Showed a dose-dependent reduction in parasitemia in infected mice. Prolonged survival of infected mice over 30 days, with a cure rate of 33%. |
| References |
| Density | 1.343g/cm3 |
|---|---|
| Boiling Point | 673.1ºC at 760 mmHg |
| Melting Point | 170-172℃ |
| Molecular Formula | C19H22O8 |
| Molecular Weight | 378.37300 |
| Flash Point | 242.7ºC |
| Exact Mass | 378.13100 |
| PSA | 125.82000 |
| LogP | 1.02690 |
| Index of Refraction | 1.573 |
| Storage condition | 20°C |
|
~16% 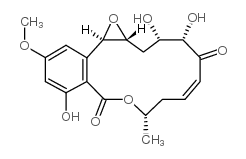
76958-67-3 |
| Literature: Selles, Patrice; Lett, Robert Tetrahedron Letters, 2002 , vol. 43, # 26 p. 4627 - 4631 |
|
~% 
76958-67-3 |
| Literature: Selles, Patrice; Lett, Robert Tetrahedron Letters, 2002 , vol. 43, # 26 p. 4627 - 4631 |
|
~% 
76958-67-3 |
| Literature: Selles, Patrice; Lett, Robert Tetrahedron Letters, 2002 , vol. 43, # 26 p. 4627 - 4631 |
|
~% 
76958-67-3 |
| Literature: Selles, Patrice; Lett, Robert Tetrahedron Letters, 2002 , vol. 43, # 26 p. 4627 - 4631 |
|
~% 
76958-67-3 |
| Literature: Selles, Patrice; Lett, Robert Tetrahedron Letters, 2002 , vol. 43, # 26 p. 4627 - 4631 |
|
~% 
76958-67-3 |
| Literature: Selles, Patrice; Lett, Robert Tetrahedron Letters, 2002 , vol. 43, # 26 p. 4627 - 4631 |
|
~% 
76958-67-3 |
| Literature: Selles, Patrice; Lett, Robert Tetrahedron Letters, 2002 , vol. 43, # 26 p. 4627 - 4631 |
| Precursor 2 | |
|---|---|
| DownStream 0 | |