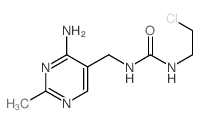
42471-28-3
| Name | nimustine |
|---|---|
| Synonyms |
EINECS 255-838-1
NIMUSTINE 1-(2-chloroethyl)-1-nitroso-3-[(2-methyl-4-aminopyrimidine-5-yl)methyl]urea 3-[(4-amino-2-methylpyrimidin-5-yl)methyl]-1-(2-chloroethyl)-1-nitrosourea 3-(4-amino-2-methyl-pyrimidin-5-ylmethyl)-1-(2-chloro-ethyl)-1-nitroso-urea MFCD01676942 Nimustina Nimustin Nimustinum [INN-Latin] 1-(2-chloro-ethyl)-3-(4-amino-2-methyl-5-pyrimidinyl)methyl-1-nitrosourea Nimustina [INN-Spanish] Nimustine [INN] Nimustinum |
| Description | Nimustine is a nitrosourea alkylating agent. Nimustine induces cell apoptosis, and activates DNA damage response and MAPK signaling. Nimustine shows anti-cancer effects, it can be used for the research of cancer[1][2]. |
|---|---|
| Related Catalog | |
| In Vitro | Nimustine (50 μM; 72-120 h) causes cell death by inducing cell apoptosis[1]. Nimustine (50 μM; 24-96 h) activates the DNA damage response pathway[1]. Nimustine (50 μM; 24-120 h) activates MAPK signaling in glioma cells[1]. Apoptosis Analysis[1] Cell Line: LN-229 cell line Concentration: 50 μM Incubation Time: 72-120 hours Result: Time-dependently induced apoptosis. Western Blot Analysis[1] Cell Line: LN-229 and glioma cell lines Concentration: 50 μM Incubation Time: 24-120 hours Result: Induced cleavage of caspase-8 and -9 and the effector caspase-3. Increased phosphorylation of ERK kinase and H2AX. Decreased phosphorylation of JNK |
| In Vivo | Nimustine (15 four times a week and 30 mg/kg twice with an interval of 2 weeks; i.v.) effectively inhibits tumor growth and the higher dose is more effective[2]. Animal Model: Female C3H/HeN mice with solid FM3A tumors[2] Dosage: 15 and 30 mg/kg Administration: Intravenous injection; 15 (4 times a week) and 30 mg/kg (twice with an interval of 2 weeks) Result: The intermittent large-dose injections resulted in better inhibition of tumor growth than did the fractionated small-dose injections. |
| Density | 1.52g/cm3 |
|---|---|
| Molecular Formula | C9H13ClN6O2 |
| Molecular Weight | 272.69200 |
| Exact Mass | 272.07900 |
| PSA | 113.57000 |
| LogP | 1.77110 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Hazard Codes | T: Toxic; |
|---|---|
| Risk Phrases | R25 |
| Safety Phrases | 36/37/39-45 |
| RIDADR | UN 2811 6.1/PG 3 |
| WGK Germany | 3 |
| RTECS | YR8450000 |
| HS Code | 2933599090 |
|
~% 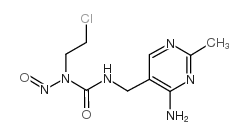
42471-28-3 |
| Literature: Sankyo Company Limited Patent: US4003901 A1, 1977 ; |
| Precursor 1 | |
|---|---|
| DownStream 0 | |
| HS Code | 2933599090 |
|---|---|
| Summary | 2933599090. other compounds containing a pyrimidine ring (whether or not hydrogenated) or piperazine ring in the structure. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |