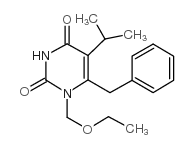
149950-60-7
| Name | emivirine |
|---|---|
| Synonyms |
I-EBU
1EtOMe6Bz5i-Pr-U Emivirine Emivirine [USAN:INN] [14C]-Emivirine 1-(ethoxymethyl)-5-(1-methylethyl)-6-(phenylmethyl)pyrimidine-2,4(1H,3H)-dione Coactinon Mkc 442 1-ethoxymethyl-5-isopropyl-6-benzyluracil 6-benzyl-1-(ethoxymethyl)-5-isopropyl uracil 6-benzyl-1-(ethoxymethyl)-5-propan-2-ylpyrimidine-2,4-dione |
| Description | Emivirine (MKC-442) is a non-nucleoside reverse transcriptase inhibitors (NNRTIs) with Ki values of 0.20 and 0.01 μM for dTTP- and dGTP-dependent DNA or RNA polymerase activity, respectively. Emivirine displays potent and selective anti-human immunodeficiency virus type 1 (HIV-1) activity[1][2]. |
|---|---|
| Related Catalog | |
| In Vitro | Emivirine (EMV) is also specific for HIV-1 RT and was without effect on HIV-2[2]. Emivirine (EMV) has no obvious toxicity for human healthy cells[2]. Cell Viability Assay[2] Cell Line: Human bone marrow cells collected from normal healthy volunteers. Concentration: 0, 0.1, 1, 10, or 100 μM. Incubation Time: 14 days. Result: At concentrations of 0.1 to 10 μM, no effect on cell growth, lactic acid production, mitochondrial DNA synthesis, or mitochondrial structure was seen compared to what occurred with untreated HepG2 cells. |
| In Vivo | Tthe approximate lethal oral dose of Emivirine (EMV) for rats was ≥3 g/kg for males and 2.5 g/kg for females[2]. Animal Model: Male Sprague-Dawley rats[2]. Dosage: 50 mg/kg. Administration: Gavage. Result: The oral absorption was 68%. |
| References |
| Molecular Formula | C17H22N2O3 |
|---|---|
| Molecular Weight | 302.37 |
| Exact Mass | 302.16300 |
| PSA | 64.09000 |
| LogP | 2.24480 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| HS Code | 2933990090 |
|---|
| Precursor 8 | |
|---|---|
| DownStream 0 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |