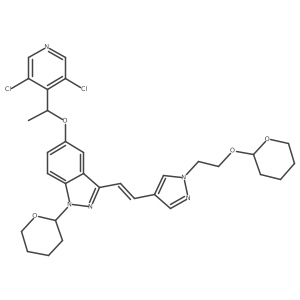
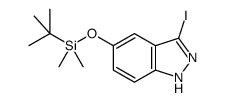
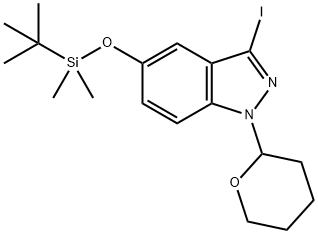
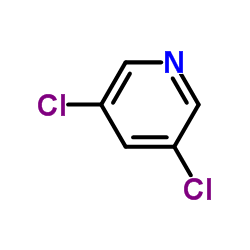
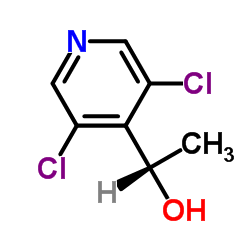
1254473-64-7
| Name | 2-[4-[(E)-2-[5-[(1R)-1-(3,5-dichloropyridin-4-yl)ethoxy]-1H-indazol-3-yl]ethenyl]pyrazol-1-yl]ethanol |
|---|---|
| Synonyms |
(R)-(E)-2-(4-(2-(5-(1-(3,5-dichloropyridin-4-yl)ethoxy)-1H-indazol-3-yl)vinyl)-1H-pyrazol-1-yl)ethanol
2-{4-[(E)-2-{5-[(1R)-1-(3,5-Dichloro-4-pyridinyl)ethoxy]-1H-indazol-3-yl}vinyl]-1H-pyrazol-1-yl}ethanol CS-0907 4-[(1E)-2-[5-[(1R)-1-(3,5-Dichloro-4-pyridinyl)ethoxy]-1H-indazol-3-yl]ethenyl]-1H-pyrazole-1-ethanol QCR-90 LY2874455 LY-2874455 |
| Description | LY2874455 is a pan-FGFR inhibitor with IC50s of 2.8, 2.6, 6.4, 6 nM for FGFR1, FGFR2, FGFR3, FGFR4, respectively. |
|---|---|
| Related Catalog | |
| Target |
FGFR1:2.8 nM (IC50) FGFR2:2.6 nM (IC50) FGFR3:6.4 nM (IC50) FGFR4:6 nM (IC50) |
| In Vitro | LY2874455 potently inhibits the Erk phosphorylation induced by FGF2 and FGF9 in both cell lines in a dose-dependent manner, with average IC50 values of 0.3 to 0.8 nM. LY2874455 indeed inhibits FGFR2 phosphorylation in SNU-16 and KATO-III cells, with estimated IC50 values of 0.8 and 1.5 nM, respectively. In addition, LY2874455 inhibits the phosphorylation of FRS2, an immediate downstream target of FGFR in these cell lines, again with a similar potency of 0.8 to 1.5 nM. Together, these results suggest that LY2874455 inhibits FGFR in the cell. The relative IC50 values of LY2874455 for KMS-11, OPM-2, L-363, and U266 cells are determined to be 0.57, 1.0, 60.4, and 290.7 nM, respectively[1]. |
| In Vivo | LY2874455 exhibits a rapid, robust, dose-dependent inhibition of tumor growth in all 4 models tested. Importantly, this molecule causes a significant regression of tumor growth in the RT-112, SNU-16, and OPM-2 tumor models, especially when dosed at 3 mg/kg twice a day. Also, LY2874455 exhibits an excellent pharmacokinetic/pharmacodynamic relationship as shown by its dose-dependent inhibition of the tumor growth at TED50 and TED90 (1 and 3 mg/kg, respectively). When tested in the RT-112 tumor xenograft model on an intermittent dosing schedule (twice a day 1 week on and 1 week off or twice a day 2 days on and 2 days off), LY2874455 is also efficacious. When rats are dosed with LY2874455 at 1 and 3 mg/kg, which is 2.6- and 7.7-fold over the TED50 (0.39 mg/kg) obtained in the rat heart IVTI assay, respectively, there are no significant changes observed in blood pressure. However, when rats are dosed with LY2874455 at 10 mg/kg, which is 25.6-fold over the TED50, there are significant increases observed in arterial pressures[1]. |
| Kinase Assay | Reaction mixtures contained 8 mM Tris-HCl (pH 7.5), 10 mM HEPES, 5 mM dithiothreitol, 10 μM ATP, 0.5 μCi 33P-ATP, 10 mM MnCl2, 150 mM NaCl, 0.01% Triton X-100, 4% DMSO, 0.05 mg/mL poly(Glu:Tyr) (4:1, average molecular weight of 20-50 kDa), and 7.5, 7.5, and 16 ng of FGFR1, FGFR3, and FGFR4, respectively, and are incubated at room temperature for 30 minutes followed by termination with 10% H3PO4. The reaction mixtures are transferred to 96-well MAFB filter plates that are washed 3 times with 0.5% H3PO4. After air-drying, the plates are read with a Trilux reader[1]. |
| Cell Assay | The different multiple myeloma cancer cell lines KMS-11 and OPM-2 cells, L-363, and U266 cells are used. Cells (2,000 per well) are first grown in RPMI for 6 hours and treated with LY2874455 at 37°C for 3 days. The cells are stained at 37°C for 4 hours and then solubilized at 37°C for 1 hour. Finally, the plate is read at 570 nm using a plate reader[1]. |
| Animal Admin | Mice[1] RT-112, OPM-2 (DSMZ), SNU-16, and NCI-H460 cells (RT-112: 2×106 per animal; OPM-2: 107 per animal; SNU-16: 106 per animal; and NCI-H460: 3×106 per animal) are mixed with Matrigel (1:1) and implanted subcutaneously into the rear flank of the mice (female, CD-1 nu/nu for RT-112, OPM-2, and NCI-H460 cells and female, severe combined immunodeficient for SNU-16 cells). The implanted tumor cells grow as solid tumors. To test the efficacy of LY2874455 in these models, the animals are orally dosed with approximately 1 mg/kg (TED50) or 3 mg/kg (TED90) of LY2874455 in 10% Acacia once (every day) or twice a day after tumors reach approximately 150 mm3. The tumor volume and body weight are measured twice a week. Rats[1] Four male rats per group are dosed with vehicle (1% hydroxyethylcellulose, 0.25% polysorbate 80, and 0.05% Dow Corning antifoam 1510-US in purified water) on day 1 and LY2874455 (1, 3, and 10 mg/kg) on day 0. On day 1, at least 120 minutes of control data are collected following vehicle administration. On day 0, data are collected for approximately 20 hours beginning after the last animal is dosed. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 672.6±55.0 °C at 760 mmHg |
| Molecular Formula | C21H19Cl2N5O2 |
| Molecular Weight | 444.314 |
| Flash Point | 360.5±31.5 °C |
| Exact Mass | 443.091583 |
| PSA | 88.85000 |
| LogP | 3.88 |
| Vapour Pressure | 0.0±2.2 mmHg at 25°C |
| Index of Refraction | 1.683 |
| Hazard Codes | Xi |
|---|
|
~41% 
1254473-64-7 |
| Literature: ELI LILLY AND COMPANY Patent: US2012/83511 A1, 2012 ; Location in patent: Page/Page column 3 ; |
|
~% 
1254473-64-7 |
| Literature: US2012/83511 A1, ; |
|
~% 
1254473-64-7 |
| Literature: US2012/83511 A1, ; |
|
~% 
1254473-64-7 |
| Literature: US2012/83511 A1, ; |
|
~% 
1254473-64-7 |
| Literature: US2012/83511 A1, ; |
|
~% 
1254473-64-7 |
| Literature: US2012/83511 A1, ; |
|
~% 
1254473-64-7 |
| Literature: US2012/83511 A1, ; |
|
~% 
1254473-64-7 |
| Literature: US2012/83511 A1, ; |
|
~% 
1254473-64-7 |
| Literature: US2012/83511 A1, ; |
| Precursor 4 | |
|---|---|
| DownStream 0 | |