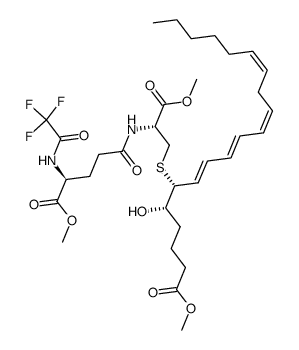
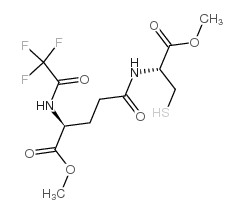
83851-42-7
| Name | leukotriene F4 |
|---|---|
| Synonyms |
(5S,6R,7E,9E,11Z,14Z)-6-{[(2S)-2-{[(4S)-4-Amino-4-carboxy-1-hydroxybutylidene]amino}-2-carboxyethyl]sulfanyl}-5-hydroxy-7,9,11,14-icosatetraenoic acid
L-γ-Glutamyl-S-[(4S,5R,6E,8E,10Z,13Z)-1-carboxy-4-hydroxy-6,8,10,13-nonadecatetraen-5-yl]-L-cysteine LTF4 Leukotriene F4 L-γ-glutamyl-S-{(1R,2E,4E,6Z,9Z)-1-[(1S)-4-carboxy-1-hydroxybutyl]pentadeca-2,4,6,9-tetraen-1-yl}-L-cysteine L-γ-Glutamyl-S-[(4S,5R,6E,8E,10Z,13Z)-1-carboxy-4-hydroxy-6,8,10,13-nonadecatetraen-5-yl]-D-cysteine |
| Description | Leukotriene F4 (LTF4), is a lipid that belongs to the Cysteinyl Leukotriene (CysTL) family[1]. Leukotriene F4 induces bronchoconstriction with an ED50 of 16 μg/kg[2]. The precursor of LTF4 is Leukotriene E4 (LTE4), which isformed from the action of a glutamyl transferase[1]. |
|---|---|
| Related Catalog | |
| Target |
Human Endogenous Metabolite |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 872.8±65.0 °C at 760 mmHg |
| Molecular Formula | C28H44N2O8S |
| Molecular Weight | 568.723 |
| Flash Point | 481.6±34.3 °C |
| Exact Mass | 568.281860 |
| PSA | 212.55000 |
| LogP | 4.54 |
| Vapour Pressure | 0.0±0.6 mmHg at 25°C |
| Index of Refraction | 1.563 |
|
~% 
83851-42-7 |
| Literature: Okuyama; Miyamoto; Shimoji; Konishi; Fukushima; Niwa; Arai; Toda; Hayashi Chemical and Pharmaceutical Bulletin, 1982 , vol. 30, # 7 p. 2453 - 2462 |
|
~% 
83851-42-7 |
| Literature: Okuyama; Miyamoto; Shimoji; Konishi; Fukushima; Niwa; Arai; Toda; Hayashi Chemical and Pharmaceutical Bulletin, 1982 , vol. 30, # 7 p. 2453 - 2462 |
|
~% 
83851-42-7 |
| Literature: Okuyama; Miyamoto; Shimoji; Konishi; Fukushima; Niwa; Arai; Toda; Hayashi Chemical and Pharmaceutical Bulletin, 1982 , vol. 30, # 7 p. 2453 - 2462 |
| Precursor 3 | |
|---|---|
| DownStream 0 | |