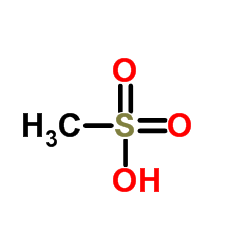
67227-57-0
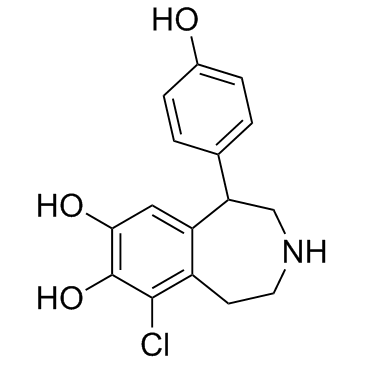
| Name | 9-chloro-5-(4-hydroxyphenyl)-2,3,4,5-tetrahydro-1H-3-benzazepine-7,8-diol,methanesulfonic acid |
|---|---|
| Synonyms |
EINECS 266-612-7
Fenoldopam monomethanesulfonate MFCD04112986 6-Chloro-2,3,4,5-tetrahydro-7,8-dihydroxy-1-(p-hydroxyphenyl)-1H-3-benzazepinium methanesulphonate Corlopam Fenoldopam methanesulfonate 6-chloro-2,3,4,5-tetrahydro-1-(4-hydroxyphenyl)-1H-3-benzazepine-7,8-diol methanesulfonate 9-chloro-5-(4-hydroxyphenyl)-2,3,4,5-tetrahydro-1H-3-benzazepine-7,8-diol Fenoldopam mesylate 6-chloro-7,8-dihydroxy-1-(p-hydroxyphenyl)-2,3,4,5-tetrahydro-1H-3-benzazepine methanesulfonate 6-chloro-2,3,4,5-tetrahydro-1-(p-hydroxyphenyl)-1H-3-benzazepine-7,8-diol methanesulfonate CORLOPAM MESYLATE Fenoldopam (mesylate) |
| Description | Fenoldopam(SKF 82526) mesylate is a drug and synthetic benzazepine derivative which acts as a selective D1 receptor partial agonist.Target: D1 ReceptorFenoldopam is a selective dopamine-1 (DA1) agonist with natriuretic/diuretic properties. Fenoldopam stimulated cAMP accumulation in LLC-PK1 cells in a dose-dependent manner, an effect which could be blocked by the DA1-selective antagonist Sch 23390. Although fenoldopam was more potent than DA (EC50 55.5 +/- 7.75 nM vs. 1.65 +/- 0.64 microM) in stimulating cAMP accumulation in LLC-PK1 cells, the maximum stimulation obtained by fenoldopam was only 37% of the maximum stimulation obtained by DA(Emax 13.0 +/- 2.95 pmol/mg of protein vs. 35.6 +/- 10.19 pmol/mg of protein) [1]. Fenoldopam is a selective dopamine1 (DA1) receptor agonist. Most of the DA1 receptor agonist activity of fenoldopam resides in the R-enantiomer, which also shows weaker alpha 2-adrenoceptor antagonist activity Fenoldopam produces vasodilation in vascular beds that are rich in vascular DA1 receptors [2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.38g/cm3 |
|---|---|
| Boiling Point | 522.6ºC at 760 mmHg |
| Molecular Formula | C17H20ClNO6S |
| Molecular Weight | 401.86 |
| Flash Point | 269.9ºC |
| PSA | 104.24000 |
| LogP | 3.77680 |
| Storage condition | room temp |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302-H317-H319 |
| Precautionary Statements | P280-P301 + P312 + P330-P305 + P351 + P338 |
| Hazard Codes | Xn |
| Risk Phrases | 22-36-42/43 |
| Safety Phrases | 22-26-36/37/39 |
| RIDADR | NONH for all modes of transport |
|
~% 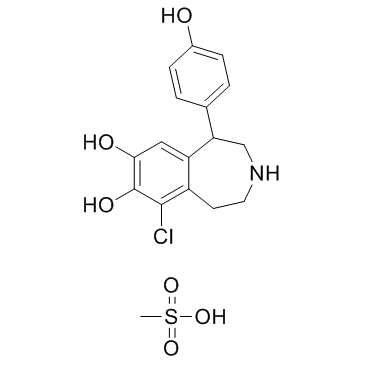
67227-57-0 |
| Literature: US2006/194967 A1, ; Page/Page column 7-8 ; |
| Precursor 2 | |
|---|---|
| DownStream 0 | |