500-62-9
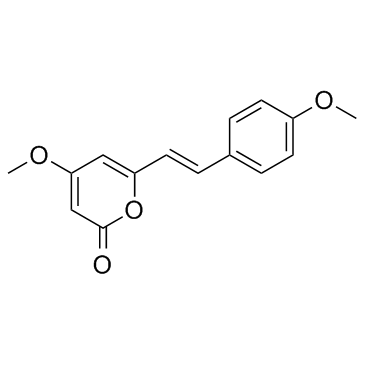
| Name | yangonin |
|---|---|
| Synonyms |
4-Methoxy-6-[(E)-2-(4-methoxyphenyl)vinyl]-2H-pyran-2-one
5-Hydroxy-3-methoxy-7-(p-methoxyphenyl)-2,4,6-heptatrienoic Acid d-Lactone 4-Méthoxy-6-[(E)-2-(4-méthoxyphényl)éthènyl]-2H-pyran-2-one 4-methoxy-6-[(E)-2-(4-methoxyphenyl)ethenyl]-2H-pyran-2-one 4-Methoxy-6-((E)-4-methoxystyryl)pyran-2-one 4-Methoxy-6-[b-(p-anisyl)vinyl]-a-pyrone 6-(p-Methoxystyryl)-4-methoxy-a-pyrone Yangonin (E)-4-Methoxy-6-[2-(4-methoxyphenyl)ethenyl]-2H-pyran-2-one |
| Description | Yangonin exhibits affinity for the human recombinant cannabinoid CB1 receptor with an IC50 and a Ki of 1.79 ± 0.53 μM and 0.72±0.21 μM, respectively. |
|---|---|
| Related Catalog | |
| Target |
CB1 Receptor:1.79 μM (IC50) CB1 Receptor:0.72 μM (Ki) RelA |
| In Vitro | Yangonin is one of the six major kavalactones found in Piper methysticum.Yangonin potently inhibits NF-κB activation through suppression of the transcriptional activity of the RelA/p65 subunit of NF-κB. Yangonin significantly inhibits the induced expression of the NF-κB-reporter gene. However, Yangonin does not interfere with TNF-α-induced inhibitor of κBα (IκBα) degradation, p65 nuclear translocation, and DNA-binding activity of NF-κB. Yangonin inhibits not only the induced NF-κB activation by overexpression of RelA/p65, but also transactivation activity of RelA/p65. Yangonin does not inhibit TNF-α-induced activation of p38, but it significantly impairs activation of ERK 1/2 and stress-activated protein kinase/JNK[2]. |
| Cell Assay | HeLa cells are seeded at 1×105 cells/mL in 96-well plates containing 100 μL of DMEM medium with 10% FBS and incubated overnight. Yangonin is dissolved in DMSO and DMSO is added to all plates to compensate the same volume of DMSO. After 24 h, the cells are pretreated with different concentrations of Yangonin (0.1-3 μM) for 1 h, followed by stimulation with or without TNF-α for 24 h. Subsequently, cells are cultured with MTT solution (5 mg/mL) for 3 h. The viable cells convert MTT to formazan, which generates a blue-purple color after dissolving in 150 μL of DMSO. The absorbance at 570 nm is measured by an ELISA plate reader[2]. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 487.6±45.0 °C at 760 mmHg |
| Melting Point | 155-157ºC |
| Molecular Formula | C15H14O4 |
| Molecular Weight | 258.269 |
| Flash Point | 219.7±28.8 °C |
| Exact Mass | 258.089203 |
| PSA | 48.67000 |
| LogP | 1.75 |
| Vapour Pressure | 0.0±1.2 mmHg at 25°C |
| Index of Refraction | 1.578 |
| Storage condition | 2-8°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| RIDADR | NONH for all modes of transport |
|---|---|
| HS Code | 2932999099 |
| Precursor 8 | |
|---|---|
| DownStream 1 | |
| HS Code | 2932999099 |
|---|---|
| Summary | 2932999099. other heterocyclic compounds with oxygen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |





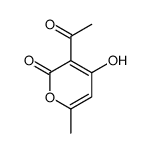

![4-methoxy-6-[2-(4-methoxyphenyl)ethyl]pyran-2-one structure](https://image.chemsrc.com/caspic/299/3155-52-0.png)