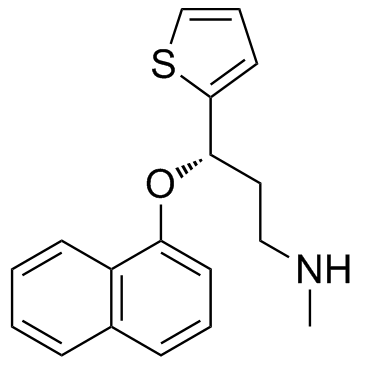
116539-59-4
| Name | (S)-duloxetine |
|---|---|
| Synonyms |
(3S)-N-methyl-3-(naphthalen-1-yloxy)-3-(thiophen-2-yl)propan-1-amine
(S)-N-Methyl-γ-(1-naphthalenyloxy)-2-thiophenepropanamine (S)-duloxetine (S)-N-Methyl-g-(1-naphthalenyloxy)-2-thiophenepropanamine (3S)-N-Methyl-3-(1-naphthyloxy)-3-(2-thienyl)-1-propanamine DULOXETIN Duloxetine (+)-(S)-N-methyl-g-(1-naphthyloxy)-2-thiophenepropylamine Intermediates Cymbalta DULOXETINE HCI (S)-N-Methyl-3-(naphthalen-1-yloxy)-3-(thiophen-2-yl)propan-1-amine DULOXETINE-D3 N-methyl-3-napthalen-1-oxy-3-thiophen-2-yl-1-amine (3S)-N-Methyl-3-(1-naphthyloxy)-3-(2-thienyl)propan-1-amine Yentreve |
| Description | Duloxetine is a serotonin-norepinephrine reuptake inhibitor with Ki of 4.6 nM, used for treatment of major depressive disorder and generalized anxiety disorder (GAD). Target: SNRIsDuloxetine inhibits the reuptake of serotonin and norepinephrine in the central nervous system. Duloxetine is also considered a less potent inhibitor of dopamine reuptake. However, duloxetine has no significant affinity for dopaminergic, adrenergic, cholinergic, histaminergic, opioid, glutamate, and GABA receptors and can therefore be considered to be a selective reuptake inhibitor at the 5-HT and NA transporters. Duloxetine undergoes extensive metabolism, but the major circulating metabolites do not contribute significantly to the pharmacologic activity. Major depressive disorder is believed to be due in part to an increase in pro-inflammatory cytokines within the central nervous system. Antidepressants including ones with a similar mechanism of action as duloxetine, i.e. serotonin metabolism inhibition, cause a decrease in proinflammatory cytokine activity and an increase in anti-inflammatory cytokines; this mechanism may apply to duloxetine in its effect on depression but research on cytokines specific to duloxetine therapy is lacking [1]. The analgesic properties of duloxetine in the treatment of diabetic neuropathy and central pain syndromes such as fibromyalgia are believed to be due to sodium ion channel blockade [2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 466.2±40.0 °C at 760 mmHg |
| Molecular Formula | C18H19NOS |
| Molecular Weight | 297.41 |
| Flash Point | 235.7±27.3 °C |
| PSA | 49.50000 |
| LogP | 3.73 |
| Vapour Pressure | 0.0±1.2 mmHg at 25°C |
| Index of Refraction | 1.628 |
| Storage condition | -20℃ |
| HS Code | 2934999090 |
|---|
| HS Code | 2934999090 |
|---|---|
| Summary | 2934999090. other heterocyclic compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |