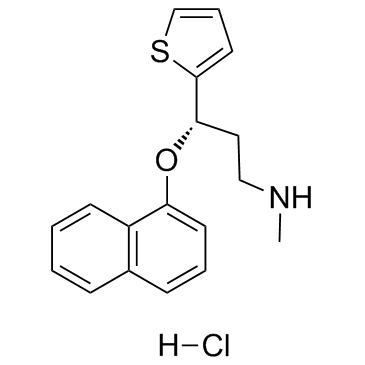
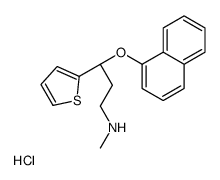
136434-34-9
| Name | (S)-duloxetine hydrochloride |
|---|---|
| Synonyms |
2-thiophenepropanamine, N-methyl-γ-(1-naphthalenyloxy)-, (γS)-, hydrochloride
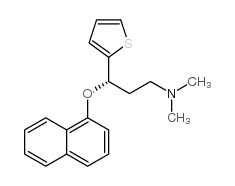
(S)-(+)-N-Methyl-3-(1-naphthyloxy)-3-(2-thienyl)propylamine Hydrochloride (+)-(S)-N-methyl-g-(1-naphthyloxy)-2-thiophenepropylamine Hydrochloride (3S)-N-méthyl-3-(naphtalén-1-yloxy)-3-thiophén-2-ylpropan-1-amine chlorhydrate (S)-Duloxetine hydrochloride (3S)-N-Methyl-3-(1-naphthyloxy)-3-(2-thienyl)propan-1-amine hydrochloride (1:1) (S)-N-Methyl-g-(1-naphthalenyloxy)-2-thiophenepropanamine Hydrochloride duloxetine hcl 2-Thiophenepropanamine, N-methyl-γ-(1-naphthalenyloxy)-, (γS)-, hydrochloride (1:1) (S)-N-Methyl-3-(naphthalen-1-yloxy)-3-(thiophen-2-yl)propan-1-amine hydrochloride (3S)-N-methyl-3-naphthalen-1-yloxy-3-thiophen-2-ylpropan-1-amine,hydrochloride (3S)-N-Methyl-3-(1-naphthyloxy)-3-(2-thienyl)-1-propanamine hydrochloride (1:1) (3S)-N-methyl-3-(naphthalen-1-yloxy)-3-thiophen-2-ylpropan-1-amine hydrochloride (3S)-N-methyl-3-(naphthalen-1-yloxy)-3-(thiophen-2-yl)propan-1-amine hydrochloride (1:1) (3S)-N-Methyl-3-(naphthalen-1-yloxy)-3-thiophen-2-ylpropan-1-aminhydrochlorid Duloxetine hydrochloride Duloxetine (hydrochloride) |
| Description | Duloxetine hydrochloride is a serotonin-norepinephrine reuptake inhibitor (SNRI) with Ki of 4.6 nM, used for treatment of major depressive disorder and generalized anxiety disorder (GAD).Target: SNRIDuloxetine (sold under the brand names Cymbalta, Ariclaim, Xeristar, Yentreve, Duzela, Dulane) is a serotonin-norepinephrine reuptake inhibitor(SNRI) manufactured and marketed by Eli Lilly. It is prescribed for major depressive disorder and generalized anxiety disorder (GAD). Duloxetine also has approval for use in osteoarthiritis and musculoskeletal pain. Duloxetine failed the US approval for stress urinary incontinence amidst concerns over liver toxicity and suicidal events; however, it was approved for this indication in Europe, where it is recommended as an add-on medication in stress urinary incontinence instead of surgery. It can also relieve the symptoms of painful peripheral neuropathy, particularly diabetic neuropathy, and it is used to control the symptoms of fibromyalgia.The main uses of duloxetine are in major depressive disorder, general anxiety disorder, stress urinary incontinence, painful peripheral neuropathy,fibromyalgia, and chronic musculoskeletal pain associated with osteoarthritis and chronic lower back pain. It is being studied for various other indications. |
|---|---|
| Related Catalog | |
| References |
| Boiling Point | 466.2ºC at 760 mmHg |
|---|---|
| Melting Point | 118-122ºC |
| Molecular Formula | C18H20ClNOS |
| Molecular Weight | 333.875 |
| Flash Point | 235.7ºC |
| Exact Mass | 333.095398 |
| PSA | 49.50000 |
| LogP | 5.82380 |
| Storage condition | -20°C Freezer |
| Water Solubility | H2O: soluble5mg/mL (clear solution, warmed) |
| Symbol |



GHS02, GHS06, GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H225-H301 + H311 + H331-H370 |
| Precautionary Statements | P210-P260-P280-P301 + P310-P311 |
| Hazard Codes | Xi: Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | 26-36/37 |
| RIDADR | UN1230 - class 3 - PG 2 - Methanol, solution |
| RTECS | XN0258000 |
| HS Code | 2934999090 |
| Precursor 6 | |
|---|---|
| DownStream 3 | |
| HS Code | 2934999090 |
|---|---|
| Summary | 2934999090. other heterocyclic compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |




![4-[3-(Methylamino)-1-(2-thienyl)propyl]-1-naphthalenol structure](https://image.chemsrc.com/caspic/202/949095-98-1.png)