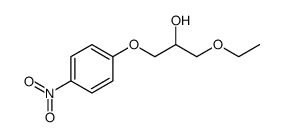
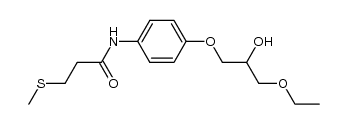
94055-76-2
| Name | Suplatast Tosylate |
|---|---|
| Synonyms |
(±)-[2-[[ p-(3-ethoxy-2-hydroxypropoxy)phenyl]carbamoyl]ethyl]dimethylsulfonium p-toluene sulfonate
[3-[[4-(3-Ethoxy-2-hydroxy propoxy)phenyl]amino]-3-oxopropyl]dimethylsulfonium salt with 4-methylbenzenesulfonic acid MFCD00867604 Suplatast tosylate (3-{[4-(3-Ethoxy-2-hydroxypropoxy)phenyl]amino}-3-oxopropyl)(dimethyl)sulfonium 4-methylbenzenesulfonate UNII:C9J89787U1 Suplatast Tosilate Suplatast (Tosilate) |
| Description | Suplatast tosilate(IPD 1151T) is a Th2 cytokine inhibitor that attenuates IL-2, IL-5 and IL-13 production and has no effect on IFN-γ production. IC50 value:Target: Th2 cytokine inhibitorSuplatast Tosilate acts as an immunoregulator that suppresses IgE production, eosinophil infiltration and histamine release. Suplatast Tosilate(IPD 1151T) exhibits antiasthmatic, anti-inflammatory and antifibrotic activity in vivo and is orally active. |
|---|---|
| Related Catalog | |
| References |
| Melting Point | 84-87ºC |
|---|---|
| Molecular Formula | C23H33NO7S2 |
| Molecular Weight | 499.641 |
| Exact Mass | 499.169830 |
| PSA | 158.67000 |
| LogP | 3.72220 |
| Storage condition | Store at RT |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| RIDADR | NONH for all modes of transport |
|---|---|
| RTECS | WR7906000 |
|
~% 
94055-76-2 |
| Literature: Journal of Medicinal Chemistry, , vol. 41, # 18 p. 3330 - 3336 |
|
~% 
94055-76-2 |
| Literature: Journal of Medicinal Chemistry, , vol. 41, # 18 p. 3330 - 3336 |
|
~% 
94055-76-2 |
| Literature: Journal of Medicinal Chemistry, , vol. 41, # 18 p. 3330 - 3336 |
|
~% 
94055-76-2 |
| Literature: Journal of Medicinal Chemistry, , vol. 41, # 18 p. 3330 - 3336 |
|
~% 
94055-76-2 |
| Literature: Journal of Medicinal Chemistry, , vol. 41, # 18 p. 3330 - 3336 |
| Precursor 5 | |
|---|---|
| DownStream 0 | |