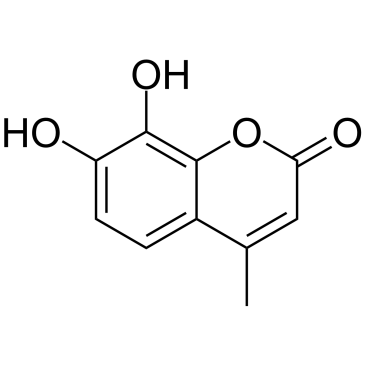
14003-96-4
| Name | 7-hydroxy-4-methyl-2-oxochromene-8-carbaldehyde |
|---|---|
| Synonyms |
7-Hydroxy-4-methyl-2-oxo-2H-1-benzopyran-8-carboxaldehyde
7-hydroxy-4-methyl-2-oxo-2H-chromen-8-carbaldehyde 7-hydroxy-4-methyl-2-oxo-2H-chromene-8-carbaldehyde 2H-1-Benzopyran-8-carboxaldehyde,7-hydroxy-4-methyl-2-oxo 8-formyl-7-hydroxy-4-methyl-coumarin 8-formyl-4-methyl-7-hydroxycoumarin 8-formyl-7-hydroxy-4-methyl-2H-<1>-benzopyran-2-one 4μ8C 8-Formyl-7-hydroxy-4-methylcoumarin |
| Description | 4μ8C (IRE1 Inhibitor III) is a small-molecule inhibitor of IRE1α. |
|---|---|
| Related Catalog | |
| In Vitro | When applies to the media of ER stressed cultured cells, 4μ8C inhibits Xbp1 splicing in a concentration-dependent manner. 4μ8C dissociates slowly from IRE1, but ishout of inhibitor leads to rapid recovery of Xbp1 splicing in cells[1].The IRE1 endoribonuclease inhibitor 4μ8c prevents the splicing of the XBP1 mRNA in response to ER stress caused by mutant proinsulin production[2]. The inositol-requiring enzyme 1α (IRE1α) is a serine-threonine kinase that plays crucial roles in activating the unfolded protein response. 4μ8C treatment dramatically inhibits IL-4 production by CD4+ T cells under Th0 conditions because both the IL-4 levels in the culture supernatant and the percentage of IL-4 positive cells are reduced by 4μ8C treatment. In addition, both IL-5 and IL-13 production are significantly reduced upon treatment with 4μ8C[3]. |
| In Vivo | 4μ8C reverses the ER stress-dependent loss of several known RIDD targets, with an EC50 of approximately 4 μM, approximating that of inhibition of XBP1 target gene activation[1]. |
| Cell Assay | INS-1 (Insulin 2 C96Y-GFP) cells (clone #4S2) cells are either left untreated or treated with 2 μg/mL doxycycline, 2 μg/mL doxycycline and 5 μM 4μ8C or 5 μM 4μ8C alone. After 48 h 50,000 cells/100 μL of media from each treatment well are seeded into a 96-well plate in duplicates. The CellTiter 96 AQueous Non-Radioactive Cell Proliferation Assay MTS is performed. The absorbance at 490 nm is then measured with a plate reader.[2]. |
| References |
| Density | 1.406±0.06 g/cm3 (20 ºC 760 Torr) |
|---|---|
| Melting Point | 189-190 ºC (ethanol ) |
| Molecular Formula | C11H8O4 |
| Molecular Weight | 204.17900 |
| Exact Mass | 204.04200 |
| PSA | 67.51000 |
| LogP | 1.61950 |
| Storage condition | -20℃ |
| Water Solubility | Very slightly soluble (0.59 g/L) (25 ºC) |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 |
| RIDADR | NONH for all modes of transport |
| HS Code | 2932209090 |
| Precursor 9 | |
|---|---|
| DownStream 2 | |
| HS Code | 2932209090 |
|---|---|
| Summary | 2932209090. other lactones. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |