129618-40-2
| Name | nevirapine |
|---|---|
| Synonyms |
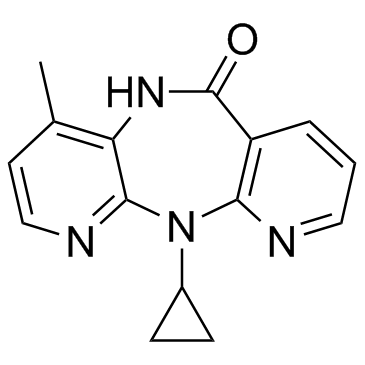
11-Cyclopropyl-4-methyl-5,11-dihydro-6H-dipyrido[3,2-b:2',3'-e][1,4]diazepin-6-one
nevirapine 11-Cyclopropyl-4-methyl-5H-dipyrido[3,2-b:2',3'-e][1,4]diazepin-6(11H)-one MFCD00866928 Viramune 11-cyclopropyl-4-methyl-5H-dipyrido[2,3-e:2',3'-f][1,4]diazepin-6-one 11-Cyclopropyl-5,11-dihydro-4-methyl-6H-dipyrido[3,2-b:2',3'-e][1,4]diazepin-6-one |
| Description | Nevirapine is a non-nucleoside inhibitor of HIV-1 reverse transcriptase used to treat and prevent HIV/AIDS; with a Ki of 270 μM. |
|---|---|
| Related Catalog | |
| Target |
Ki: 270 μM (HIV-1 reverse transcriptase)[1] |
| In Vitro | Nevirapine itself is an inhibitor of only CYP3A4 at concentrations that are well above those of therapeutic relevance (Ki=270 μM)[1]. Nevirapine has been used as a re-differentiation agent to treat cancers in several human cancer models. At all doses (100, 200, 350, 500 μM) tested, nevirapine significantly inhibits cell proliferation after 48 h treatment. At high dose (500 μM), nevirapine significantly increases the percentage of apoptotic cells compared with control[2]. Nevirapine is a potent and selective inhibitor (IC50=10-100 nM) of the replication of a wide variety of HIV-1 strains in several cellular assays[3]. |
| In Vivo | Nevirapine is available for use in combination with nucleoside HIV-1 reverse transcriptase inhibitors (e.g., zidovudine, didanosine, etc.). Nevirapine has received FDA approval for use in combination with HIV-1 protease inhibitors (e.g., saquinavir, ritonavir, indinavir, etc.). In humans, nevirapine is eliminated primarily in the urine as glucuronide conjugates of 2-, 3-, 8-, and 12-hydroxynevirapine[1]. Nevirapine is completely absorbed in both sexes of mouse, rat, rabbit, monkey, and chimpanzee. Nevirapine is extensively metabolized in both sexes of all animal species studied[4]. Nevirapine (9 mg/kg, 18 mg/kg and 36 mg/kg) shows significant reduction in ulcer severity score and ulcer index as compared to the control[5] |
| Cell Assay | FRO cells are seeded into 96-well culture plates at 10,000 cells/well. Cells are treated with different doses of nevirapine (0, 100, 200, 350 and 500 μM) for 48 h. MTT dye (5 mg/mL) is added to each well for additional 4 h, and the reaction is then stopped by the addition of DMSO. Optical density is measured at 490 nm on a multi-well plate reader[2]. |
| Animal Admin | Rats: Nevirapine and [14C] Nevirapine are dissolved together in absolute ethanol and methylene chloride (1:1, v/v) with mild heating. The concentration of drug in suspension is 2 mg/mL (20 mg/kg, 26 μCi) for oral dosing to rats and 6.7 mg/mL (20.3 mg/kg, 10 μCi males, 8.9 μCi females) for intraduodenal administration to rats before bile collection. The i.v. dose is administered to rats (1.1 mg/kg, 20 μCi) as a solution in 20% ethanol/80% saline[4]. Mice: Nevirapine and [14C] Nevirapine are dissolved together in absolute ethanol and methylene chloride (1:1, v/v) with mild heating. The concentration of drug in suspension is 2 mg/mL (20 mg/kg, 2.5 μCi) with a specific activity of 5.55 μCi/mg for oral dosing to mice[4]. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 415.4±45.0 °C at 760 mmHg |
| Melting Point | 247°C |
| Molecular Formula | C15H14N4O |
| Molecular Weight | 266.298 |
| Flash Point | 205.0±28.7 °C |
| Exact Mass | 266.116760 |
| PSA | 63.57000 |
| LogP | 2.03 |
| Vapour Pressure | 0.0±1.0 mmHg at 25°C |
| Index of Refraction | 1.672 |
| Storage condition | -20°C Freezer |
| Hazard Codes | Xi: Irritant; |
|---|---|
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36-S36/37 |
| RIDADR | UN 2206 6.1/PG 3 |
| WGK Germany | 3 |
| Packaging Group | III |
| Hazard Class | 6.1 |
| HS Code | 2934994000 |
| Precursor 9 | |
|---|---|
| DownStream 5 | |
| HS Code | 2934994000 |
|---|---|
| Summary | 2934994000. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
![7-chloro-5-cyclopropyl-9-methyl-5,10-dihydro-4,5,6,10-tetraaza-dibenzo[a,d]cyclohepten-11-one structure](https://image.chemsrc.com/caspic/136/135575-99-4.png)
![11-Cyclopropyl-5,11-dihydro-4-(hydroxyMethyl)-6H-dipyrido[3,2-b:2',3'-e][1,4]diazepin-6-one structure](https://image.chemsrc.com/caspic/452/133627-24-4.png)
![1'-cyclopropyl-4-methyl-1'H,2H-spiro[pyridine-3,2'-pyrido[2,3-d]pyrimidine]-2,4',6(1H,3'H)-trione structure](https://image.chemsrc.com/caspic/265/1350539-32-0.png)