53174-06-4
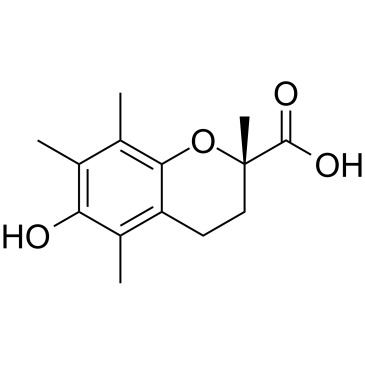
| Name | (2S)-6-hydroxy-2,5,7,8-tetramethyl-3,4-dihydrochromene-2-carboxylic acid |
|---|---|
| Synonyms |
UNII-O763YB84E3
MFCD00180756 (S)-(-)-3,4-dihydro-6-hydroxy-2,5,7,8-tetramethyl-2H-1-benzopyran-2-carboxylic acid (-)-(S)-6-hydroxy-2,5,7,8-tetramethyl-chroman-2-carboxylic acid (S)-(-)-Trolox (S)-(-)-Trolox C (S)-Trolox(TM) (2S)-6-Hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid |
| Description | (S)-Trolox is a water-soluble analogue of vitamin E, in which the phytyl chain is replaced with a carboxyl group. (S)-Trolox is frequently used as a model compound for studies of structural features, as well as a standard for evaluation of antioxidant activity. (S)-Trolox has potent and specific neuroprotective and antioxidant effects[1][2]. |
|---|---|
| Related Catalog | |
| In Vitro | The neuroprotective efficacy of antioxidant molecules against iodoacetate (IAA) neurotoxicity in rat cerebellar granule cell (CGC) cultures is investigated. In the absence of MK-801, (S)-Trolox displays marginal neuroprotective effects. In the presence of MK-801 (10 μM), the neuroprotective efficacy of (S)-Trolox is greatly enhanced, giving rise to a recovery in MTT-reductase activity equivalent to 80–100% of control cultures. (S)-Trolox displays EC50 value of 78 μM. The fluorescence increase in IAA-stimulated DCFH-DA-loaded cultures is inhibited in a dose-dependent manner by the antioxidants (S)-Trolox with an IC50 value of 97 μM. The antioxidant (S)-Trolox demonstrate apotent and specific neuroprotective action in an in vitro model of neurodegeneration induced by inhibition of the glycolytic enzyme GAPDH[1]. |
| References |
| Density | 1.219g/cm3 |
|---|---|
| Boiling Point | 450.3ºC at 760 mmHg |
| Melting Point | 160ºC (dec.)(lit.) |
| Molecular Formula | C14H18O4 |
| Molecular Weight | 250.29000 |
| Flash Point | 171ºC |
| Exact Mass | 250.12100 |
| PSA | 66.76000 |
| LogP | 2.48570 |
| Index of Refraction | 1.567 |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi |
| Risk Phrases | 36/37/38 |
| Safety Phrases | 26-37/39 |
| RIDADR | NONH for all modes of transport |
| HS Code | 2932999099 |
| HS Code | 2932999099 |
|---|---|
| Summary | 2932999099. other heterocyclic compounds with oxygen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |