61379-65-5
| Name | rifapentine |
|---|---|
| Synonyms |
rifamycin-S
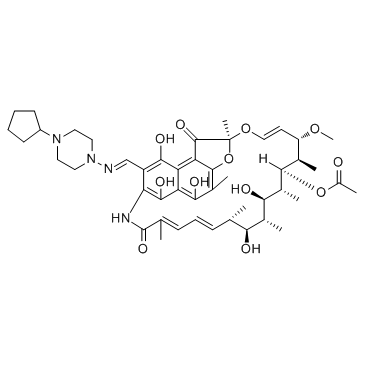
Rifamycin-B-tripropyl-hydrazid (7S,9E,11S,12R,13S,14R,15R,16R,17S,18S,19E,21Z)-26-{[(4-Cyclopentyl-1-piperazinyl)imino]methyl}-2,15,17,27,29-pentahydroxy-11-methoxy-3,7,12,14,16,18,22-heptamethyl-6,23-dioxo-8,30-dioxa-24-azatetracy ;clo[23.3.1.1.0]triaconta-1(29),2,4,9,19,21,25,27-octaen-13-yl acetate Rifapentine rifamycin-B tripropylhydrazide EINECS 262-743-9 |
| Description | Rifapentine (Priftin; DL 473) is an antibiotic compound used in the treatment of tuberculosis.Target: AntibacterialRifapentine inhibits DNA-dependent RNA polymerase activity in susceptible cells. Specifically, it interacts with bacterial RNA polymerase but does not inhibit the mammalian enzyme. A review of alternative regimens for prevention of active tuberculosis in HIV-negative individuals with latent TB found that a weekly, directly observed regimen of rifapentine with isoniazid for three months was as effective as a daily, self -administered regimen of isoniazid for nine months. But the rifapentine-isoniazid regimen had higher rates of treatment completion and lower rates of hepatotoxicity . However the rates of treatment-limiting adverse events were higher in the rifapentine-isoniazid regimen [1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 969.3±65.0 °C at 760 mmHg |
| Melting Point | 179-180ºC |
| Molecular Formula | C47H64N4O12 |
| Molecular Weight | 877.031 |
| Flash Point | 540.0±34.3 °C |
| Exact Mass | 876.452087 |
| PSA | 216.66000 |
| LogP | 2.58 |
| Vapour Pressure | 0.0±0.3 mmHg at 25°C |
| Index of Refraction | 1.625 |
| Storage condition | -20°C Freezer |
| Water Solubility | methanol: soluble2mg/mL, clear, red to red-brown |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Hazard Codes | Xi |
| Risk Phrases | 36/37/38 |
| Safety Phrases | 26 |
| RIDADR | NONH for all modes of transport |
| RTECS | JQ0902000 |
| HS Code | 2941903000 |
| HS Code | 2941903000 |
|---|