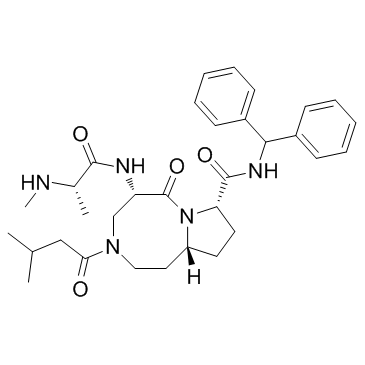
1071992-99-8
| Name | (5S,8S,10aR)-N-benzhydryl-5-[[(2S)-2-(methylamino)propanoyl]amino]-3-(3-methylbutanoyl)-6-oxo-1,2,4,5,8,9,10,10a-octahydropyrrolo[1,2-a][1,5]diazocine-8-carboxamide |
|---|---|
| Synonyms |
sm 406
at406 (5S,8S,10aR)-N-(Diphenylmethyl)-5-[(N-methyl-L-alanyl)amino]-3-(3-methylbutanoyl)-6-oxodecahydropyrrolo[1,2-a][1,5]diazocine-8-carboxamide qcr-136 at-406 AT 406 |
| Description | AT-406 is a potent and orally bioavailable Smac mimetic and an antagonist of IAPs, and it binds to XIAP, cIAP1, and cIAP2 proteins with Ki of 66.4, 1.9, and 5.1 nM, respectively. |
|---|---|
| Related Catalog | |
| Target |
Ki: 66.4 nM (XIAP), 1.9 nM (cIAP1), 5.1 nM (cIAP2) |
| In Vitro | AT-406 mimic closely the AVPI peptide in both hydrogen bonding and hydrophobic interactions with XIAP, with additional hydrophobic contacts with W323 of XIAP. AT-406 is more sensitive to these IAPs than Smac AVPI peptide with 50-100 fold binding affinities. AT-406 (1 μM) completely restores the activity of caspase-9, which is suppressed by 500 nM XIAP BIR3 in a cell-free system. In MDA-MB-231 cell, AT-406 induces rapid cellular cIAP1 degradation and also pulls down the cellular XIAP protein. AT-406 effectively inhibits lots of human cancer cell lines and shows IC50 of 144 and 142 nM in MDA-MB-231 cell and SK-OV-3 ovarian cell, with low toxicity against normal-like human breast epithelial MCF-12F cells and primary human normal prostate epithelial cells. AT-406 induces apoptosis in MDA-MB-231 cell by inducing activation of caspase-3 and cleavage of PARP[1]. AT-406 displays single agent activity in ovarian cancer cell lines. The IC50 values of AT-406 in these ovarian cancer cells range from 0.05-0.5 µg/mL. AT-406 exhibits anti-ovarian cancer efficacy both as a single agent and in combination with carboplatin. AT-406 (30 μg/mL) induced degradation of XIAP in the drug sensitive ovarian cancer cell lines[2]. |
| In Vivo | AT-406 has good pharmacokinetic properties and oral bioavailability in mice, rats, non-human primates, and dogs. In the MDA-MB-231 xenograft, AT-406 effectively induces cIAP1 degradation and processing of procaspase-8, cleavage of PARP in tumor tissues at 100 mg/kg with well toleration even at 200 mg/kg. AT-406 induces significant tumor growth inhibition with p of 0.0012 at 100 mg/kg[2]. SM-406 (30, 100 mg/kg, p.o.) decreases the plasma and tumor in tumor-bearing mice[3]. |
| Kinase Assay | MDA-MB-231 cell lysates are prepared by solubilizing cells in ice cold buffer containing KCl (50 mM), EGTA (5 mM), MgCl2 (2 mM) DTT (1 mM), 0.2% CHAPS and HEPES, (50 mM, pH 7.5), containing cocktail protease inhibitors, incubating on ice for 10 minutes, then freezing in liquid nitrogen. Cytochrome c and dATP are added to the cell lysates, which are then incubated at 30°C in a water bath for 60 minutes to activate caspase-9. Addition of recombinant XIAP BIR3 protein dose-dependently suppresses the activity of caspase-9. Different concentrations of a tested Smac mimetic (1 nM-100 μM) are added to determine the restoration of the activity of these caspases. |
| Cell Assay | Cells are seeded in 96-well flat bottom cell culture plates at a density of 3-4 ×103 cells/well with AT-406 and incubated for 4 days. The rate of cell growth inhibition after treatment with different concentrations of AT-406 is determined by assaying with (2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium monosodium salt (WST-8). WST-8 is added to each well to a final concentration of 10%, and then the plates are incubated at 37°C for 2−3 hours. The absorbance of the samples is measured at 450 nm using a TECAN ULTRA reader. Concentration of AT-406 that inhibits cell growth by 50% (IC50) is calculated by comparing absorbance in the untreated cells and the cells treated with AT-406. |
| Animal Admin | SCID mice (8-10 per group) bearing MDA-MB-231 xenograft tumors are treated with different doses of compound 2, or 7.5 mg/kg of Taxotere or vehicle control daily, 5 days a week for 2 weeks. Tumor sizes and animal weights are measured 3 times a week during the treatment and twice a week after the treatment. Data are presented as mean tumor volumes±SEM. Statistical analyses are performed by two-way ANOVA and unpaired two-tailed t test, using Prism. P < 0.05 is considered statistically significant. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 840.5±65.0 °C at 760 mmHg |
| Molecular Formula | C32H43N5O4 |
| Molecular Weight | 561.715 |
| Flash Point | 462.1±34.3 °C |
| Exact Mass | 561.331482 |
| PSA | 117.83000 |
| LogP | 2.09 |
| Vapour Pressure | 0.0±3.1 mmHg at 25°C |
| Index of Refraction | 1.603 |
| Storage condition | -20℃ |