71079-19-1
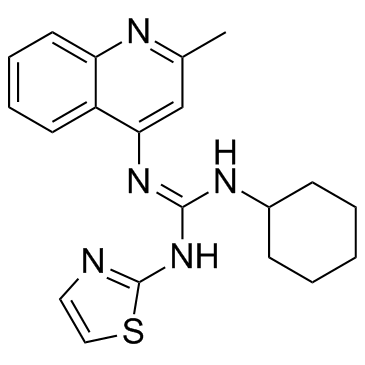
| Name | 2-cyclohexyl-1-(2-methylquinolin-4-yl)-3-(1,3-thiazol-2-yl)guanidine |
|---|---|
| Synonyms |
1-Cyclohexyl-2-(2-methyl-4-quinolyl)-3-(2-thiazolyl)guanidine
Guanidine,1-cyclohexyl-2-(2-methyl-4-quinolyl)-3-(2-thiazolyl) EINECS 275-184-0 Timegadinum [INN-Latin] N-cyclohexyl-N'-(2-methyl-quinolin-4-yl)-N''-thiazol-2-yl-guanidine N-cyclohexyl-N''-4-(2-methylquinolyl)-N'-2-thiazolylguanidine Timegadine Timegadina [INN-Spanish] Guanidine,N-cyclohexyl-N'-(2-methyl-4-quinolinyl)-N''-2-thiazolyl |
| Description | Timegadine, a new antiinflammatory agent, is found to be a potent, competitive inhibitor of cyclo-oxygenase (COX) and lipo-oxygenase, with IC50s ranging from 5 nM (washed rabbit platelets) to 20 μM (rat brain) for COX and 100 μM for lipo-oxygenase both in the cytosol fraction of horse platelet homogenates, and in washed rabbit platelets. |
|---|---|
| Related Catalog | |
| Target |
COX:5 nM (IC50, in rabbit platelets) COX:20 μM (IC50, in rat brain) lipo-oxygenase:100 μM (IC50, in horse and washed rabbit platelets) |
| In Vitro | Timegadine, a new antiinflammatory agent, is found to be a potent, competitive inhibitor of of COX and lipo-oxygenase, with IC50s ranging from 5 nM (washed rabbit platelets) to 20 μM (rat brain) for COX and 100 μM for lipo-oxygenase both in the cytosol fraction of horse platelet homogenates, and in washed rabbit platelets[2]. |
| In Vivo | Timegadine, a new antiinflammatory agent, is found to be a potent, competitive inhibitor of prostaglandin synthetase which also inhibits cyclo-oxygenase (COX) and lipoxygenase. Daily oral doses of 10 to 30 mg/kg of Timegadine significantly inhibit both the primary and secondary lesions of adjuvant arthritis when the treatment is initiated on the day of the disease induction and continues for 28 days. Timegadine is able specifically to prevent the development of the swelling of the non-injected paw until 28 days after the adjuvant injection when administered for 5 days prior to and 5 days after the induction of the disease, in analogy with the effect of cyclophosphamide[1]. |
| Animal Admin | Female inbred Lewis rats (body weight ~150 g) are used in adjuvant arthritis and experimental allergic encephalomyelitis experiments. Paw volume is determined by mercury displacement plethismometer. Timegadine is administered orally in a volume of 1 mL/kg body weight, suspended in 0.5% carboxymethylcellulose[1]. |
| References |
| Density | 1.31 g/cm3 |
|---|---|
| Boiling Point | 537.6ºC at 760 mmHg |
| Molecular Formula | C20H23N5S |
| Molecular Weight | 365.49500 |
| Flash Point | 278.9ºC |
| Exact Mass | 365.16700 |
| PSA | 90.44000 |
| LogP | 5.48550 |
| Index of Refraction | 1.701 |
| Storage condition | 2-8℃ |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|