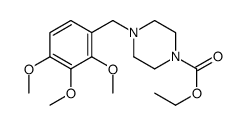
5011-34-7
| Name | 1-[(2,3,4-trimethoxyphenyl)methyl]piperazine |
|---|---|
| Synonyms |
Trimetazidine HCl
EINECS 225-690-2 MFCD00868263 Trimetazidine 1-(2,3,4-Trimethoxybenzyl)(2,2,3,3,5,5,6,6-H)piperazine |
| Description | Trimetazidine is a selective long chain 3-ketoyl coenzyme A thiolase inhibitor with an IC50 of 75 nM, which can inhibit β-oxidation of free fatty acid (FFA). Trimetazidine is an effective antianginal agent and a cytoprotective drug, has anti-oxidant, anti-inflammatory, antinociceptive and gastroprotective properties. Trimetazidine triggers autophagy. Trimetazidine is also a 3-hydroxyacyl-CoA dehydrogenase (HADHA) inhibitor[1][2][3][4]. |
|---|---|
| Related Catalog | |
| Target |
IC50: 75 nM (long chain 3-ketoyl coenzyme A thiolase)[2] β-oxidation[2] Autophagy[3] 3-hydroxyacyl-CoA dehydrogenase (HADHA)[4] |
| In Vitro | Trimetazidine (1-100 μM; 24 hours; HUVECs) could enhance the viability of the injured HUVECs induced by oxidation in a certain dose-dependent manner[1]. Cell Viability Assay[1] Cell Line: Human umbilical vein endothelial cells (HUVECs) Concentration: 1 μM,10 μM,100 μM Incubation Time: 24 hours Result: Enhanced the viability of the injured HUVECs induced by oxidation. |
| In Vivo | Trimetazidine (5-20 mg/kg; oral administration; 1 hour; Swiss albino male mice) in 10 and 20mg/kg doses significantly raises the seizure-threshold current in the ICES test in the mice[5]. Animal Model: Swiss albino male mice (24-35 g)[4] Dosage: 5 mg/kg, 10 mg/kg and 20 mg/kg; 10 mL/kg body weight Administration: Oral administration ; 1 hour Result: In 10 and 20mg/kg doses significantly raised the seizure-threshold current in the ICES test. |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 364.0±37.0 °C at 760 mmHg |
| Melting Point | 200 - 205ºC |
| Molecular Formula | C14H22N2O3 |
| Molecular Weight | 274.385 |
| Flash Point | 174.0±26.5 °C |
| Exact Mass | 274.213257 |
| PSA | 42.96000 |
| LogP | 0.80 |
| Vapour Pressure | 0.0±0.8 mmHg at 25°C |
| Index of Refraction | 1.524 |
| Hazard Codes | Xi |
|---|---|
| HS Code | 2933599090 |
|
~% 
5011-34-7 |
| Literature: Ferte, Jacques; Kuehnel, Jean-Marc; Chapuis, Genevieve; Rolland, Yves; Lewin, Guy; Schwaller, Marc A. Journal of Medicinal Chemistry, 1999 , vol. 42, # 3 p. 478 - 489 |
|
~% 
5011-34-7 |
| Literature: Ferte, Jacques; Kuehnel, Jean-Marc; Chapuis, Genevieve; Rolland, Yves; Lewin, Guy; Schwaller, Marc A. Journal of Medicinal Chemistry, 1999 , vol. 42, # 3 p. 478 - 489 |
| Precursor 2 | |
|---|---|
| DownStream 0 | |
| HS Code | 2933599090 |
|---|---|
| Summary | 2933599090. other compounds containing a pyrimidine ring (whether or not hydrogenated) or piperazine ring in the structure. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |