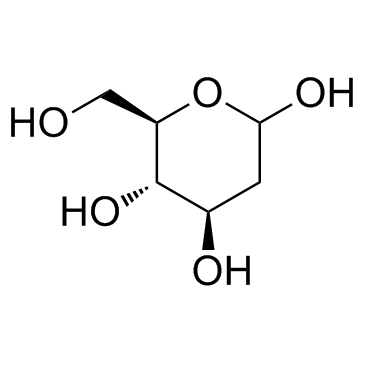
CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
MQ3325000
-
CHEMICAL NAME :
-
D-arabino-Hexose, 2-deoxy-
-
CAS REGISTRY NUMBER :
-
154-17-6
-
BEILSTEIN REFERENCE NO. :
-
1723331
-
LAST UPDATED :
-
199701
-
DATA ITEMS CITED :
-
6
-
MOLECULAR FORMULA :
-
C6-H12-O5
-
MOLECULAR WEIGHT :
-
164.18
-
WISWESSER LINE NOTATION :
-
VH1YQYQYQ1Q -D-ARABINO
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
250 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
APFRAD Annales Pharmaceutiques Francaises. (SPPIF, B.P.22, F-41353 Vineuil, France) V.1- 1943- Volume(issue)/page/year: 39,327,1981
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
2 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
JPPMAB Journal of Pharmacy and Pharmacology. (Pharmaceutical Soc. of Great Britain, 1 Lambeth High St., London SEI 7JN, UK) V.1- 1949- Volume(issue)/page/year: 17,814,1965 ** REPRODUCTIVE DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
2 gm/kg
-
SEX/DURATION :
-
female 7-8 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants)
-
REFERENCE :
-
85DJA5 "Malformations Congenitales des Mammiferes," Tuchmann-Duplessis, H., Paris, Masson et Cie, 1971 Volume(issue)/page/year: -,95,1971
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
4 gm/kg
-
SEX/DURATION :
-
female 7-14 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus)
-
REFERENCE :
-
85DJA5 "Malformations Congenitales des Mammiferes," Tuchmann-Duplessis, H., Paris, Masson et Cie, 1971 Volume(issue)/page/year: -,95,1971
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
2 gm/kg
-
SEX/DURATION :
-
female 7-10 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants) Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus) Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
REFERENCE :
-
TJADAB Teratology, The International Journal of Abnormal Development. (Alan R. Liss, Inc., 41 E. 11th St., New York, NY 10003) V.1- 1968- Volume(issue)/page/year: 40,143,1989
|



![[2-oxo-2-(1H-pyrrol-2-yl)ethyl] benzoate structure](https://image.chemsrc.com/caspic/122/5729-75-9.png)