182167-02-8
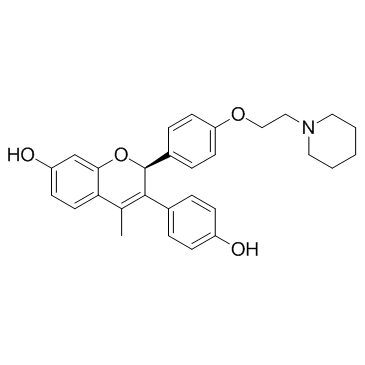
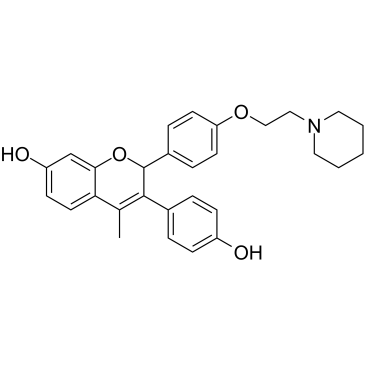
| Name | (2S)-3-(4-Hydroxyphenyl)-4-methyl-2-{4-[2-(1-piperidinyl)ethoxy]p henyl}-2H-chromen-7-ol hydrochloride (1:1) |
|---|---|
| Synonyms |
(S)-3-(4-carboxybenzyl)willardiine
(aS)-a-Amino-3-((4-carboxyphenyl)methyl)-3,4-dihydro-2,4-dioxo-1(2H)-pyrimidinepropanoicacid (S)-3-(4-hydroxyphenyl)-4-methyl-2-[4-[2-(1-piperidinyl)ethoxy]phenyl]-2H-1-benzopyran-7-ol UBP 282 |
| Description | Acolbifene (EM652) is a fourth-generation selective estrogen receptor antagonist with a LC50 value of 22±3 nM. |
|---|---|
| Related Catalog | |
| References |
| Boiling Point | 651.5ºC at 760mmHg |
|---|---|
| Molecular Formula | C29H31NO4 |
| Molecular Weight | 457.56 |
| Flash Point | 347.8ºC |
| PSA | 62.16000 |
| LogP | 6.76680 |
| Precursor 6 | |
|---|---|
| DownStream 0 | |