88426-33-9
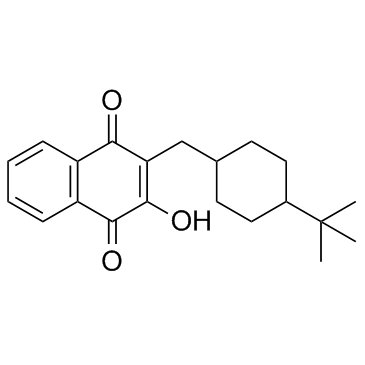
| Name | 3-[(4-tert-butylcyclohexyl)methyl]-4-hydroxynaphthalene-1,2-dione |
|---|---|
| Synonyms |
2-[(4-tert-butylcyclohexyl)methyl]-3-hydroxynaphthalene-1,4-dione
2-[[4-(1,1-Dimethylethyl)cyclohexyl]methyl]-3-hydroxy-1,4-naphthalenedione Butalex UNII-0354RT7LG4 2-[(4-tert-Butylcyclohexyl)methyl]-3-hydroxynaphthalen-1,4-dion 2-((4-tert-Butylcyclohexyl)methyl)-3-hydroxy-1,4-naphthochinon BW 720C Buparvaquone Buparvaquonum [Latin] Buparvacuona [Spanish] 3-(4-t-Butylcyclohexyl)methyl-2-hydroxy-1,4-naphthoquinone MFCD01712789 2-((4-(1,1-Dimethylethyl)cyclohexyl)methyl)-3-hydroxy-1,4-naphthalenedione 2-[(4-tert-Butylcyclohexyl)methyl]-3-hydroxy-1,4-naphthoquinone 2-Hydroxy-3-{[4-(2-methyl-2-propanyl)cyclohexyl]methyl}-1,4-naphthoquinone 2-((4-tert-Butylcyclohexyl)methyl)-3-hydroxy-1,4-naphthoquinone |
| Description | Buparvaquone is a hydroxynaphthoquinone antiprotozoal drug related to parvaquone and atovaquone. |
|---|---|
| Related Catalog | |
| In Vitro | In 4-day proliferation assays, buparvaquone efficiently inhibits N. caninum tachyzoite replication(IC50=4.9 nM; IC100=100 nM)[1]. Buparvaquone is significantly selective against L. (L.) infantum chagasi intracellular amastigotes, with an IC50 value of 1.5 μM. Other cutaneous species are also susceptible to buparvaquone, with IC50 values in the range 1-4 μM[2]. |
| In Vivo | Treatment of N. caninum infected mice with buparvaquone (100 mg/kg) either by intraperitoneal injection or gavage prevents neosporosis symptoms in 4 out of 6 mice in the intraperitoneally treated group, and in 6 out of 7 mice in the group receiving oral treatment[1]. Both a hydrous gel and water-in-oil emulsion of buparvaquone significantly reduce cutaneous parasite burden and lesion size, compared with the untreated control[3]. |
| Cell Assay | To study whether pretreatment of host cells prior to invasion had any effect on parasite proliferation, confluent HFF grown in 6-well plates are treated with 1 µM buparvaquone in medium for 1 h or 5 h, and controls are exposed to the corresponding amounts of DMSO. Subsequently, the drug-containing medium is removed and monolayers are ished 4 times with Hank's Balanced Salt Solution, and are infected with Nc-Liv tachyzoites in 5 mL medium without any drug or solvent. After 2 days, cells are collected with a cell scraper, centrifuged, ished once more in PBS, and the pellet is stored at −20 °C prior to quantification of N. caninum proliferation by N. caninum-specific real time PCR as outlined below[1]. |
| Animal Admin | Mice: On day 0, all mice are infected by intraperitoneal (i.p.) injection of freshly purified N. caninum tachyzoites. After 48 h, mice receive BPQ (100 mg/kg) as suspension in corn oil either by i.p. injection of a volume of 100 µl or by oral application of 100 µl by gavage. The control groups obtained the corresponding amount of the solvent only, either i.p. or orally (see Table 2). The treatments are performed 5 times on a daily basis. If not indicated otherwise, mice are inspected twice daily for clinical signs (ruffled coat, apathy, hind limb paralysis) until day 21 post infection (p.i.), at which time they are euthanized[1]. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 460.7±45.0 °C at 760 mmHg |
| Melting Point | 178-184ºC |
| Molecular Formula | C21H26O3 |
| Molecular Weight | 326.429 |
| Flash Point | 246.5±25.2 °C |
| Exact Mass | 326.188202 |
| PSA | 54.37000 |
| LogP | 6.45 |
| Vapour Pressure | 0.0±1.2 mmHg at 25°C |
| Index of Refraction | 1.574 |
| Storage condition | Refrigerator |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| RIDADR | NONH for all modes of transport |
|---|---|
| RTECS | QJ5766600 |