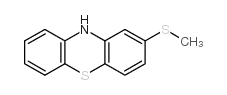
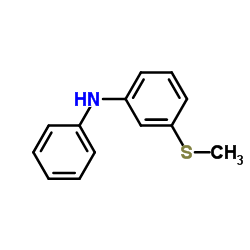
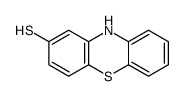
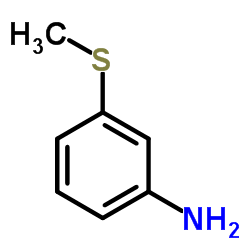
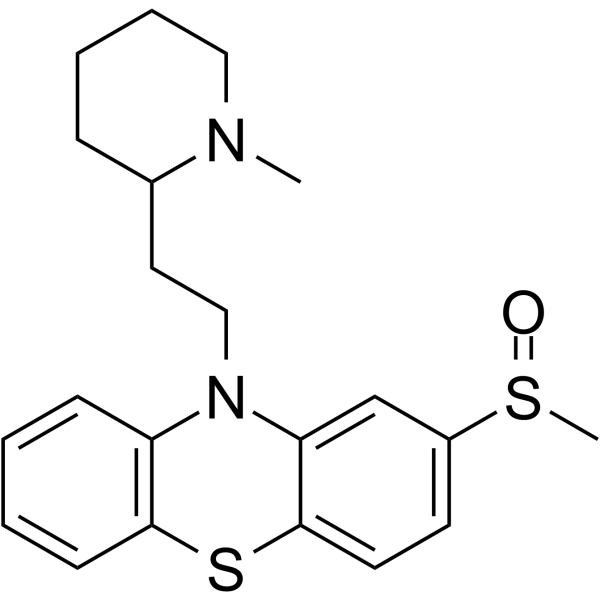
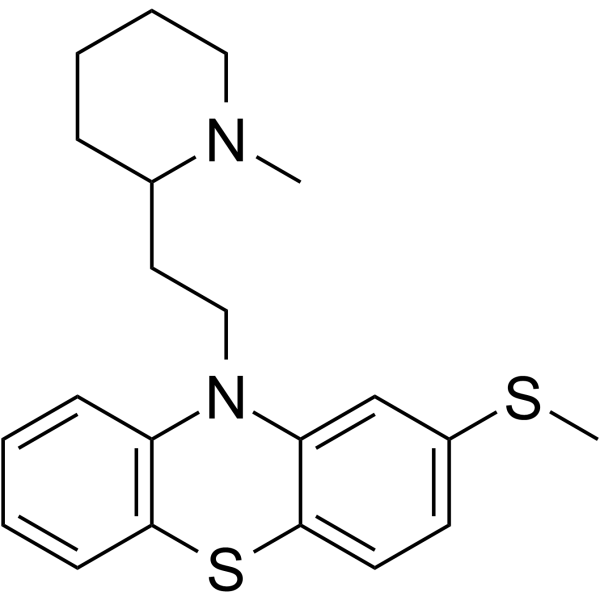
50-52-2
| Name | thioridazine |
|---|---|
| Synonyms |
3-Methylmercapto-N-[2'-(N'-methyl-2-piperidyl)ethyl]phenothiazine
EINECS 200-044-2 MFCD00242875 2-Methylmercapto-10-[2-(N-methyl-2-piperidyl)ethyl]phenothiazine 10-[2-(1-methylpiperidin-2-yl)ethyl]-2-methylsulfanylphenothiazine 10-[2-(1-methylpiperidin-2-yl)ethyl]-2-(methylthio)-10H-phenothiazine THIORIDAZINE 10-[2-(1-Methyl-2-piperidinyl)ethyl]-2-(methylsulfanyl)-10H-phenothiazine 10-[2-(1-Methyl-2-piperidinyl)ethyl]-2-(methylthio)-10H-phenothiazine 10-[2-(1-Methylpiperidin-2-yl)ethyl]-2-(methylsulfanyl)-10H-phenothiazine UNII:N3D6TG58NI |
| Description | Thioridazine, an antagonist of the dopamine receptor D2 family proteins, exhibits potent anti-psychotic and anti-anxiety activities. Thioridazine is also a potent inhibitor of PI3K-Akt-mTOR signaling pathways with anti-angiogenic effect. Thioridazine shows antiproliferative and apoptosis induction effects in various types of cancer cells, with specificity on targeting cancer stem cells (CSCs)[1][2][3][4]. |
|---|---|
| Related Catalog | |
| In Vitro | Thioridazine (0.01-100 μM; 48 h) reduces the cell viability of NCI-N87 and AGS cells in a concentration-dependent manner[2]. Thioridazine (15 μM; 24 h) reduces cell viability of the cervical (HeLa, Caski and C33A) and endometrial (HEC-1-A and KLE) cancer cells[4]. Thioridazine (1-15 μM; 24-48 h) induces gastric cancer cell death via the mitochondrial apoptosis pathway and mitochondrial pathway[2]. Thioridazine (15 μM; 24 h) modulates the regulation of cell cycle progression by interfering with the PI3K/Akt pathway and induces G1 cell cycle arrest in cervical and endometrial cancer cells [4]. Thioridazine inhibits the growth of antibiotic-sensitive and multidrug-resistant strains of A. baumannii[3]. Cell Viability Assay[1] Cell Line: NCI-N87 and AGS cells. Concentration: 0.01, 0.1, 0.5, 1, 5, 10, 20, 50, 100 μM. Incubation Time: 48 hours. Result: Exhibited cytotoxicity in gastric cancer cells. Western Blot Analysis[1] Cell Line: NCI-N87 and AGS cells Concentration: 1, 5, 10, 15 μM. Incubation Time: 24, 48 hours. Result: Downregulated the precursors of caspase-9, caspase-8 and caspase-3. |
| In Vivo | Thioridazine (25 mg/kg; i.p. every 3 days for 3 weeks) extends the survival of tumor-bearing mice and reduces the number of pluripotent embryonal carcinoma (EC) cells within tumors[5]. Thioridazine (1.0-5.0 mg/kg; s.c.) reduces oral behavior and selectively blocks repetitive head bobbing[1]. Animal Model: Nude and Rag2KO mice were injected with iPS cells or NT2D1 cells[5] Dosage: 25 mg/kg. Administration: I.p. every 3 days for 3 weeks. Result: Reduced the number of OCT4-expressing cells within malignant teratocarcinomas and extended the survival of tumor-bearing mice. With no effect on fertility. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 515.7±50.0 °C at 760 mmHg |
| Melting Point | 72-74° |
| Molecular Formula | C21H26N2S2 |
| Molecular Weight | 370.574 |
| Flash Point | 265.7±30.1 °C |
| Exact Mass | 370.153748 |
| PSA | 57.08000 |
| LogP | 6.13 |
| Vapour Pressure | 0.0±1.3 mmHg at 25°C |
| Index of Refraction | 1.677 |
| Storage condition | ?20°C |
| Water Solubility | Practically insoluble in water, very soluble in methylene chloride, freely soluble in methanol, soluble in ethanol (96 per cent). |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |



GHS02, GHS06, GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H225-H301 + H311 + H331-H370-H412 |
| Precautionary Statements | P210-P260-P273-P280-P301 + P310-P311 |
| Hazard Codes | F,T |
| Risk Phrases | 11-23/24/25-39/23/24/25-52/53 |
| Safety Phrases | 16-36/37-45-61 |
| RIDADR | UN1230 - class 3 - PG 2 - Methanol, solution |
|
~% 
50-52-2 |
| Literature: Bourquin et al. Helvetica Chimica Acta, 1958 , vol. 41, p. 1072,1080 |
|
~% 
50-52-2 |
| Literature: Bourquin et al. Helvetica Chimica Acta, 1958 , vol. 41, p. 1072,1080 |
|
~% 
50-52-2 |
| Literature: Bourquin et al. Helvetica Chimica Acta, 1958 , vol. 41, p. 1072,1080 |
|
~% 
50-52-2 |
| Literature: Bourquin et al. Helvetica Chimica Acta, 1958 , vol. 41, p. 1072,1080 |
|
~% 
50-52-2 |
| Literature: Bourquin et al. Helvetica Chimica Acta, 1958 , vol. 41, p. 1072,1080 |
|
~% 
50-52-2 |
| Literature: Bourquin et al. Helvetica Chimica Acta, 1958 , vol. 41, p. 1072,1080 |
|
~% 
50-52-2 |
| Literature: Bourquin et al. Helvetica Chimica Acta, 1958 , vol. 41, p. 1072,1080 |
| Precursor 6 | |
|---|---|
| DownStream 3 | |