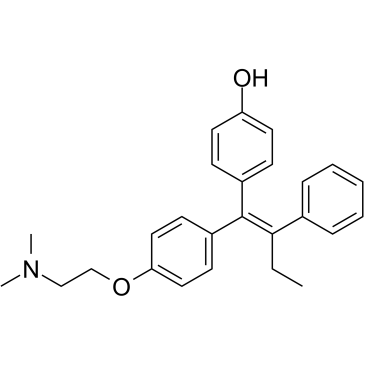
68047-06-3
| Name | afimoxifene |
|---|---|
| Synonyms |
MFCD00278780
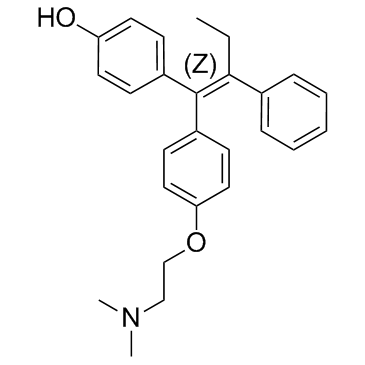
4-hydroxytamoxifen 4-[(1Z)-1-{4-[2-(Dimethylamino)ethoxy]phenyl}-1-phenyl-1-buten-2-yl]phenol 4-{(1Z)-1-[{4-[2-(dimethylamino)ethoxy]phenyl}(phenyl)methylidene]propyl}phenol 4-{(1Z)-1-[{4-[2-(dimethylamino)ethoxy]phenyl}(phenyl)methylene]propyl}phenol 4-hydroxy-tamoxifen |
| Description | 4-Hydroxytamoxifen is a selective estrogen receptor modulator (SERM). |
|---|---|
| Related Catalog | |
| Target |
Estrogen receptor:3.3 nM (IC50) CRISPR/Cas9 |
| In Vitro | 4-Hydroxytamoxifen (Monohydroxytamoxifen) is a selective oestrogen receptor antagonist, with an IC50 of 3.3 nM for the [3H]oestradiol binding to oestrogen receptor. 4-Hydroxytamoxifen (10, 100 nM) enables to inhibit the binding of [3H]oestradiol to the human 8 S oestrogen receptor[1]. 4-Hydroxytamoxifen activates intein-linked inactive Cas9, reduces off-target CRISPR-mediated gene editing. In human cells, conditionally active Cas9s modify target genomic sites with up to 25-fold higher specificity than wild-type Cas9[2]. |
| In Vivo | 4-Hydroxytamoxifen (0.2, 1 and 5 μg/day, p.o.) causes a dose-related decrease in uterine wet weight of immature rats[1]. 4-Hydroxytamoxifen (6 μg/0.1 mL sesame oil/day, s.c.) effectively attenuates methamphetamine-induced nigrostriatal dopamine depletions in bothsexes of intact and gonadectomized C57BL/6 J mice. 4-Hydroxytamoxifen does not alter the dopamine content levels in the striatum[3]. |
| Kinase Assay | Cytosol (200 μL) is incubated for 30 min at 4°C with different concentrations of oestradiol, tamoxifen and (4-Hydroxytamoxifen) or dihydroxytamoxifen administered in 10 μL methanol. Control tubes are incubated with 10 μL methanol alone and non-specific binding is determined in a parallel incubation of cytosol (200 μL) with methanol (10 μL) containing DES (5 × 106 M). [2,4,6,7-3H]Oestradiol solution (50 μL) in TED buffer is added to each tube to give a final concentration of 2 × 10-9 M. Incubation is continued for 4 h (4°C) and then 400 μL of a suspension of dextran-coated charcoal (250 mg % Norit A, 2.5 mg % dextran) in TED buffer are added and allowed to stand for 20 min. Tubes are centrifuged at 800 g for 10 min (4°C) and 400 μL samples of the supernatant are added to 10 mL tritium scintillator (6 g butyl PBD, 135 mL toluene, 720 ml dioxan, 100 g naphthalene, 45 mL absolute methanol). Samples are counted for 10 min in a liquid scintillation spectrometer. Counting efficiency is determined by external standardization (35-36 %). Results are represented as a percentage of the specifically bound radioactivity (c.p.m.) in the control tubes[1]. |
| Animal Admin | Mice[3] Animals of each sex are divided into two groups: one group receives 4-Hydroxytamoxifen [6 μg/0.1 mL sesame oil/day, subcutaneously (s.c.) starting at 06.00 h] injections for three consecutive days, while the other group receives an equivalent amount of sesame oil injection for 3 days. Four hours following the third injection, each group is then subdivided into two groups: one receives four cumulative doses of methamphetamine hydrochloride (10 mg/kg, s.c.), and the other receives a comparable volume of saline at 2-h intervals. Bilateral gonadectomy is performed under pentobarbital anesthesia (50 mg/kg, intraperitoneally). Five weeks after surgery,gonadectomized mice of each sex are randomly divided into six groups. Five groups of each sex receive three daily injections ofvarious concentrations of 4-Hydroxytamoxifen (0, 1.5, 3.0, 6.0, and 12.0 μg/0.1 mL sesame oil/day). Four hours following the third injection, mice receive four doses of methamphetamine (MA, 10 mg/kg) at 2-h intervals. The remaining group of each sex receives sesame oil pretreatment for three consecutive days, followed by saline injections, and serves as the control group[3]. |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 514.4±50.0 °C at 760 mmHg |
| Melting Point | 105-107ºC |
| Molecular Formula | C26H29NO2 |
| Molecular Weight | 387.514 |
| Flash Point | 264.9±30.1 °C |
| Exact Mass | 387.219818 |
| PSA | 32.70000 |
| LogP | 7.34 |
| Vapour Pressure | 0.0±1.4 mmHg at 25°C |
| Index of Refraction | 1.597 |
| Storage condition | 2-8°C |
| Stability | Stable. Store cool. Incompatible with strong oxidizing agents. |
| Water Solubility | 95% ethanol: 20 mg/mL |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |


GHS07, GHS08 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302-H312-H332-H361 |
| Precautionary Statements | P280 |
| Personal Protective Equipment | Eyeshields;full-face particle respirator type N100 (US);Gloves;respirator cartridge type N100 (US);type P1 (EN143) respirator filter;type P3 (EN 143) respirator cartridges |
| Hazard Codes | Xn |
| Risk Phrases | R20/21/22;R63 |
| Safety Phrases | S22-S23-S36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | SL1210000 |
| Precursor 8 | |
|---|---|
| DownStream 2 | |
![(E)-1-[4-(benzyloxy)phenyl]-1-[4-(2-dimethylaminoethoxy)phenyl]-2-phenylbut-1-ene structure](https://image.chemsrc.com/caspic/436/109517-76-2.png)

![(E,Z)-1-[4-(benzyloxy)phenyl]-1-(4-hydroxyphenyl)-2-phenylbut-1-ene structure](https://image.chemsrc.com/caspic/447/185223-78-3.png)
![(E)-1-[4-(benzyloxy)phenyl]-1-(4-hydroxyphenyl)-2-phenylbut-1-ene structure](https://image.chemsrc.com/caspic/396/440646-09-3.png)
![4-benzyloxy-4'-[(p-perfluorotolyl)oxy]benzophenone structure](https://image.chemsrc.com/caspic/196/1028310-31-7.png)
![[4-[(E)-1-[4-[2-(dimethylamino)ethoxy]phenyl]-2-phenylbut-1-enyl]phenyl] acetate structure](https://image.chemsrc.com/caspic/362/76117-70-9.png)