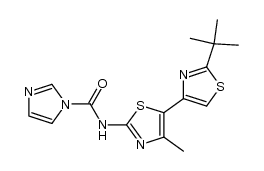
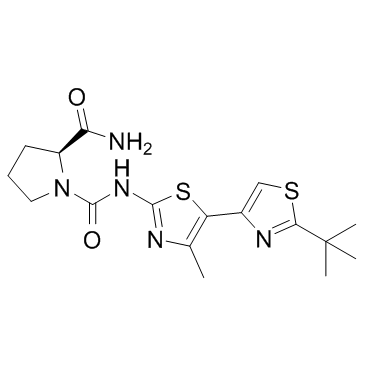
1166227-08-2
| Name | (2S)-1-N-[5-(2-tert-butyl-1,3-thiazol-4-yl)-4-methyl-1,3-thiazol-2-yl]pyrrolidine-1,2-dicarboxamide |
|---|---|
| Synonyms |
cc-500
A-66 (S)-pyrrolidine-1,2-dicarboxylic acid 2-amide 1-[(2-tert-butyl-4'-methyl-[4,5']bithiazolyl-2'-yl)-amide] CS-0477 (2S)-N-[4'-Methyl-2-(2-methyl-2-propanyl)-4,5'-bi-1,3-thiazol-2'-yl]-1,2-pyrrolidinedicarboxamide A66 |
| Description | A66 is a highly specific and selective p110α inhibitor with an IC50 of 32 nM. |
|---|---|
| Related Catalog | |
| Target |
p110α:32 nM (IC50) p110α E545K:30 nM (IC50) p110α H1047R:43 nM (IC50) p110γ:3480 nM (IC50) PI3K-C2β:462 nM (IC50) PI4Kβ:236 nM (IC50) |
| In Vitro | A66 is a potent inhibitor of the wild-type and oncogenic forms of p110α but not other class-I PI3K isoforms[1]. The p110α-specific inhibitor A66 (0.7 μM) induces a 75-80% reduction in focus formation by the highly transforming iSH2 mutants KS459delN, DKRMNS560del, and K379E. The p110α-specific inhibitor A66 reduced phosphorylation of Akt on T308 by all p85 mutants[2]. |
| In Vivo | The optimal dosing strategy for xenograft studies is determined by investigating the drug pharmacokinetics after a dose of 10 mg/kg of body weight by intraperitoneal injection in CD-1 mice. Despite a short half-life of only 0.42 h, the large Cmax (8247 nM) of A66 S that is reached 30 min after dosing ensured that the AUC0-inf (area under the curve from zero time to infinity) (6809 nM•h) is similar to that of BEZ-235 (7333 nM•h), which has a longer half-life of 2.73 h. Furthermore, the A66 on SK-OV-3 tumour tissue is tested using a single dose of 100 mg/kg of body weight to determine whether a long-lasting effect of the drug could be achieved on target tissues. These studies show that A66 causes a profound reduction in the phosphorylation of Akt/PKB and p70 S6 kinase, but not of ERK (extracellular-signal-regulated kinase), at both 1 and 6 h after dosing. Levels of A66 in plasma are determined to be 21.1±1.2 μM and 9.1±1.1 μM at 1 and 6 h after drug injection, whereas levels of A66 in the tumor are 22.7±2.1 μM and 16.0±1.3 μM at the same time points[1]. |
| Kinase Assay | IC50 values are evaluated using the PI3K (human) HTRF Assay. p85α/p110δ is obtained from Invitrogen. All other isoforms are produced in-house by co-expressing full-length human p85α with the indicated human full-length catalytic subunit containing a histidine tag at the N-terminus to allow purification. The PI3Ks are titrated and used at a concentration between their EC65-EC80 values. PI3K activity in immunoprecipitates is assayed using an antibody to the N-SH2 (N-Src homology 2) domain of p85α. Assays for other lipid kinases and protein kinases are performed by the National Centre for Protein Kinase Profiling and Invitrogen Drug Discovery Services[1]. |
| Animal Admin | Mice[1] Age-matched specific pathogen-free Rag1-/- or NIH-III mice are subcutaneously inoculated on the right flank with 5×106 U87MG, SK-OV-3 or HCT-116 cells in PBS. Tumour diameter as measured by electronic calipers is used to calculate tumour volume (mm3) based on the formula (L×w2)×π/6 (where L=longest tumour diameter and w=perpendicular diameter). A66 is administered in 20% 2-hydroxypropyl-β-cyclodextrin in water, whereas BEZ-235 is administered in 10% ethanol. Control mice are administered the A66 dosing vehicle alone. The drugs are dosed by intraperitoneal injection as the free base equivalent at a dosing volume of 10 mL/kg of body weight. For tumour pharmacodynamic studies, mice are administered a single dose of A66 or the control vehicle when tumors reached approximately 8-9 mm in diameter. Animals are killed 1 or 6 h after dosing and the tumors are removed, biopulverized and assayed for protein concentration. For antitumor efficacy studies, dosing began when tumors are well established, averaging approximately 7 mm in diameter. Doses are administered once daily (QD) or twice daily (BID) with injections separated by a minimum of approximately 8 h. Different dosing schedules are used for the three xenograft models depending on the rate of tumor growth and the body weight tolerance of control mice. Animals are dosed daily for 21 days or twice daily for 16 days (SK-OV-3), daily for 14 days (U87MG) and daily for 7 days (HCT-116). Animals are monitored daily for any signs of emerging toxicity and body weight is recorded. Mice are killed if they developed moderate signs of toxicity or if body weight loss exceeded 20% of starting weight. TGI (tumour growth inhibition) is calculated on the final day of dosing by determining the relative tumour size of drug-treated mice as a percentage of the average relative tumour size of control mice. The statistical significance of TGI values is determined by one-way ANOVA with Bonferroni multiple comparison analysis using GraphPad Prism 5.02. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Molecular Formula | C17H23N5O2S2 |
| Molecular Weight | 393.527 |
| Exact Mass | 393.129303 |
| PSA | 162.17000 |
| LogP | 0.63 |
| Index of Refraction | 1.640 |
| Storage condition | -20℃ |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 |
| Precautionary Statements | P301 + P312 + P330 |
| RIDADR | NONH for all modes of transport |
| HS Code | 29341000 |
|
~% 
1166227-08-2 |
| Literature: WO2009/80705 A2, ; Page/Page column 52 ; WO 2009/080705 A2 |
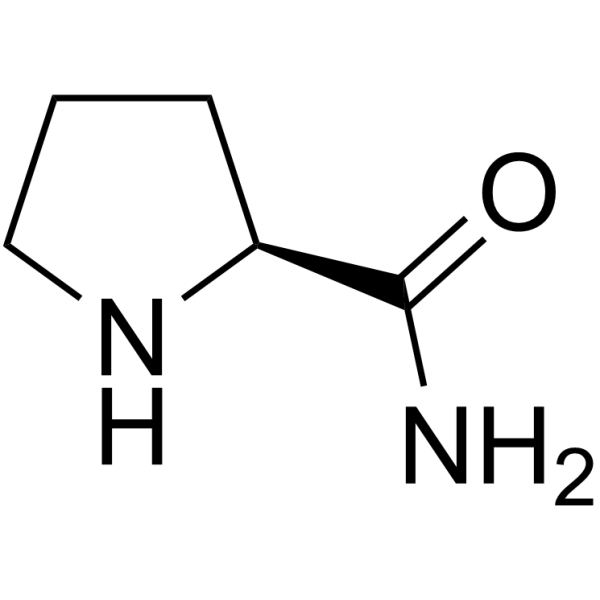
| Precursor 2 | |
|---|---|
| DownStream 0 | |