3877-86-9
| Name | cucurbitacin D |
|---|---|
| Synonyms |
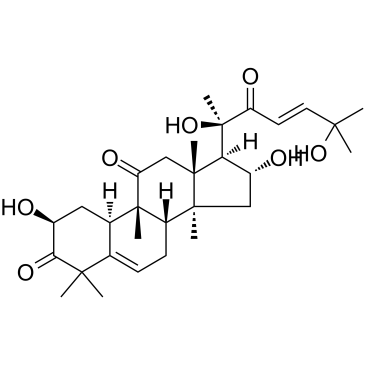
(2S,4R,9β,16α,23E)-2,16,20,25-Tetrahydroxy-9,10,14-trimethyl-4,9-cyclo-9,10-secocholesta-5,23-diene-1,11,22-trione
(2S,8S,9R,10R,13R,14S,16R,17R)-17-[(E,2R)-2,6-dihydroxy-6-methyl-3-oxohept-4-en-2-yl]-2,16-dihydroxy-4,4,9,13,14-pentamethyl-2,7,8,10,12,15,16,17-octahydro-1H-cyclopenta[a]phenanthrene-3,11-dione Elatericine A Elatericin A (2S,8S,9R,10R,13R,14S,16R,17R)-17-[(2R,4E)-2,6-Dihydroxy-6-methyl-3-oxo-4-hepten-2-yl]-2,16-dihydroxy-4,4,9,13,14-pentamethyl-7,8,9,10,12,13,14,15,16,17-decahydro-1H-cyclopenta[a]phenanthrene-3,11(2H,4H)-dione Cucurbitacin D (2S,4R,8ξ,9β,16α,23E)-2,16,20,25-Tetrahydroxy-9,10,14-trimethyl-4,9-cyclo-9,10-secocholesta-5,23-diene-1,11,22-trione (2S,8S,9R,10R,13R,14S,16R,17R)-17-[(2R,4E)-2,6-Dihydroxy-6-methyl-3-oxo-4-hepten-2-yl]-2,16-dihydroxy-4,4,9,13,14-pentamethyl-7,8,9,10,12,13,14,15,16,17-decahydro-1H-cyclopenta[a]phenanthren-3,11(2H,4H)-dion |
| Description | Cucurbitacin D is an active component in Cucurbita texana, disrupts interactions between Hsp90 and two co-chaperones, Cdc37 and p23. Cucurbitacin D prevents Hsp90 client (Her2, Raf, Cdk6, pAkt) maturation without induction of the heat shock response. Anti-cancer activity[1]. |
|---|---|
| Related Catalog | |
| Target |
HSP90 |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 684.0±55.0 °C at 760 mmHg |
| Melting Point | 151-152ºC |
| Molecular Formula | C30H44O7 |
| Molecular Weight | 516.666 |
| Flash Point | 381.4±28.0 °C |
| Exact Mass | 516.308716 |
| PSA | 132.13000 |
| LogP | 1.48 |
| Vapour Pressure | 0.0±4.8 mmHg at 25°C |
| Index of Refraction | 1.582 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Hazard Codes | Xi: Irritant; |
|---|---|
| HS Code | 29144000 |