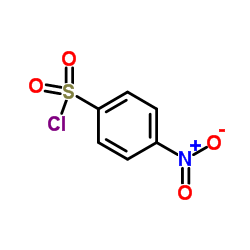
144-82-1
| Name | sulfamethizole |
|---|---|
| Synonyms |
MFCD00053363
4-Amino-N-(5-methyl-1,3,4-thiadiazol-2-yl)benzenesulfonamide Sulfamethizole Sulfarine EINECS 205-641-1 Sulphamethizole |
| Description | Sulfamethizole is a sulfathiazole antibacterial agent.Target: AntibacterialSulfamethizole is a sulfathiazole antibacterial agent. Sulfamethizole is a competitive inhibitor of bacterial para-aminobenzoic acid (PABA), a substrate of the enzyme dihydropteroate synthetase. The inhibited reaction is necessary in these organisms for the synthesis of folic acid. Sulfamethizole, an inhibitor of dihydropteroate synthetase and the formation of folic acid, inhibited bioluminescence more than growth [1]. Treatment with sulfamethizole resulted in a significant reduction in bacterial counts in all samples from a susceptible strain (MIC, 128 micro g/ml) and a resistant strain (MIC, 512 micro g/ml). Infection with a sulII gene-positive strain (MIC, >2,048 micro g/ml) could not be treated with sulfamethizole, as no effect could be demonstrated in the urine, bladder, or kidneys [2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Boiling Point | 504.9±52.0 °C at 760 mmHg |
| Melting Point | 210 °C |
| Molecular Formula | C9H10N4O2S2 |
| Molecular Weight | 270.331 |
| Flash Point | 259.1±30.7 °C |
| Exact Mass | 270.024506 |
| PSA | 134.59000 |
| LogP | 0.51 |
| Vapour Pressure | 0.0±1.3 mmHg at 25°C |
| Index of Refraction | 1.687 |
| Storage condition | 2-8°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H317 |
| Precautionary Statements | P280 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Faceshields;Gloves |
| Hazard Codes | Xi: Irritant; |
| Risk Phrases | R43 |
| Safety Phrases | S36/37 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 2 |
| RTECS | WP0875000 |
| HS Code | 2935009090 |
|
~71% 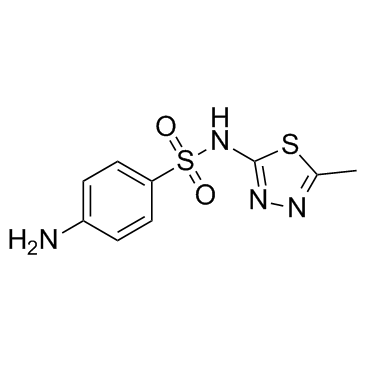
144-82-1 |
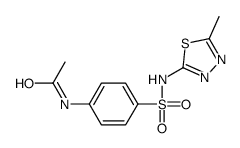
| Literature: Ahad, Ali Md.; Zuohe, Song; Du-Cuny, Lei; Moses, Sylvestor A.; Zhou, Li Li; Zhang, Shuxing; Powis, Garth; Meuillet, Emmanuelle J.; Mash, Eugene A. Bioorganic and Medicinal Chemistry, 2011 , vol. 19, # 6 p. 2046 - 2054 |
|
~99% 
144-82-1 |
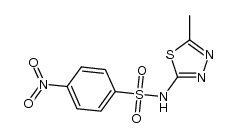
| Literature: PRESIDENT AND FELLOWS OF HARVARD COLLEGE; SHARPE, Arlene, H.; BUTTE, Manish, J.; OYAMA, Shinji Patent: WO2011/82400 A2, 2011 ; Location in patent: Page/Page column 52 ; |
|
~% 
144-82-1 |
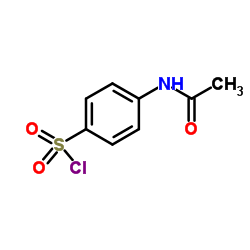
| Literature: Recueil des Travaux Chimiques des Pays-Bas, , vol. 62, p. 207 Archiv der Pharmazie (Weinheim, Germany), , vol. 284, p. 53,57 |
|
~% 
144-82-1 |
| Literature: WO2011/82400 A2, ; |
|
~% 
144-82-1 |
| Literature: CH230860 , ; |
|
~% 
144-82-1 |
| Literature: US2447702 , ; |
|
~% 
144-82-1 |
| Literature: US2358031 , ; CH231190 , ; |
|
~% 
144-82-1 |
| Literature: DE840544 , ; DRP/DRBP Org.Chem. |
| Precursor 7 | |
|---|---|
| DownStream 4 | |
| HS Code | 2935009090 |
|---|---|
| Summary | 2935009090 other sulphonamides VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:6.5% General tariff:35.0% |


![Benzenesulfonamide,4-[2-(2,6-diamino-3-pyridinyl)diazenyl]-N-(5-methyl-1,3,4-thiadiazol-2-yl) structure](https://image.chemsrc.com/caspic/087/29817-74-1.png)
![Benzenesulfonamide, 4-[2-(8-hydroxy-5-quinolinyl)diazenyl]-N-(5-methyl-1,3,4-thiadiazol-2-yl) structure](https://image.chemsrc.com/caspic/368/29821-96-3.png)
![Benzenesulfonamide,4-[2-(4,5-dihydro-3-methyl-5-oxo-1-phenyl-1H-pyrazol-4-yl)diazenyl]-N-(5-methyl-1,3,4-thiadiazol-2-yl) structure](https://image.chemsrc.com/caspic/057/29822-02-4.png)