Sulfamethizole
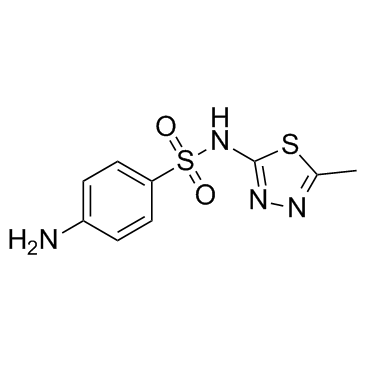
Sulfamethizole structure
|
Common Name | Sulfamethizole | ||
|---|---|---|---|---|
| CAS Number | 144-82-1 | Molecular Weight | 270.331 | |
| Density | 1.6±0.1 g/cm3 | Boiling Point | 504.9±52.0 °C at 760 mmHg | |
| Molecular Formula | C9H10N4O2S2 | Melting Point | 210 °C | |
| MSDS | Chinese USA | Flash Point | 259.1±30.7 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of SulfamethizoleSulfamethizole is a sulfathiazole antibacterial agent.Target: AntibacterialSulfamethizole is a sulfathiazole antibacterial agent. Sulfamethizole is a competitive inhibitor of bacterial para-aminobenzoic acid (PABA), a substrate of the enzyme dihydropteroate synthetase. The inhibited reaction is necessary in these organisms for the synthesis of folic acid. Sulfamethizole, an inhibitor of dihydropteroate synthetase and the formation of folic acid, inhibited bioluminescence more than growth [1]. Treatment with sulfamethizole resulted in a significant reduction in bacterial counts in all samples from a susceptible strain (MIC, 128 micro g/ml) and a resistant strain (MIC, 512 micro g/ml). Infection with a sulII gene-positive strain (MIC, >2,048 micro g/ml) could not be treated with sulfamethizole, as no effect could be demonstrated in the urine, bladder, or kidneys [2]. |
| Name | sulfamethizole |
|---|---|
| Synonym | More Synonyms |
| Description | Sulfamethizole is a sulfathiazole antibacterial agent.Target: AntibacterialSulfamethizole is a sulfathiazole antibacterial agent. Sulfamethizole is a competitive inhibitor of bacterial para-aminobenzoic acid (PABA), a substrate of the enzyme dihydropteroate synthetase. The inhibited reaction is necessary in these organisms for the synthesis of folic acid. Sulfamethizole, an inhibitor of dihydropteroate synthetase and the formation of folic acid, inhibited bioluminescence more than growth [1]. Treatment with sulfamethizole resulted in a significant reduction in bacterial counts in all samples from a susceptible strain (MIC, 128 micro g/ml) and a resistant strain (MIC, 512 micro g/ml). Infection with a sulII gene-positive strain (MIC, >2,048 micro g/ml) could not be treated with sulfamethizole, as no effect could be demonstrated in the urine, bladder, or kidneys [2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Boiling Point | 504.9±52.0 °C at 760 mmHg |
| Melting Point | 210 °C |
| Molecular Formula | C9H10N4O2S2 |
| Molecular Weight | 270.331 |
| Flash Point | 259.1±30.7 °C |
| Exact Mass | 270.024506 |
| PSA | 134.59000 |
| LogP | 0.51 |
| Vapour Pressure | 0.0±1.3 mmHg at 25°C |
| Index of Refraction | 1.687 |
| InChIKey | VACCAVUAMIDAGB-UHFFFAOYSA-N |
| SMILES | Cc1nnc(NS(=O)(=O)c2ccc(N)cc2)s1 |
| Storage condition | 2-8°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H317 |
| Precautionary Statements | P280 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Faceshields;Gloves |
| Hazard Codes | Xi: Irritant; |
| Risk Phrases | R43 |
| Safety Phrases | S36/37 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 2 |
| RTECS | WP0875000 |
| HS Code | 2935009090 |
|
~71% 
Sulfamethizole CAS#:144-82-1 |
| Literature: Ahad, Ali Md.; Zuohe, Song; Du-Cuny, Lei; Moses, Sylvestor A.; Zhou, Li Li; Zhang, Shuxing; Powis, Garth; Meuillet, Emmanuelle J.; Mash, Eugene A. Bioorganic and Medicinal Chemistry, 2011 , vol. 19, # 6 p. 2046 - 2054 |
|
~99% 
Sulfamethizole CAS#:144-82-1 |
| Literature: PRESIDENT AND FELLOWS OF HARVARD COLLEGE; SHARPE, Arlene, H.; BUTTE, Manish, J.; OYAMA, Shinji Patent: WO2011/82400 A2, 2011 ; Location in patent: Page/Page column 52 ; |
|
~% 
Sulfamethizole CAS#:144-82-1 |
| Literature: Recueil des Travaux Chimiques des Pays-Bas, , vol. 62, p. 207 Archiv der Pharmazie (Weinheim, Germany), , vol. 284, p. 53,57 |
|
~% 
Sulfamethizole CAS#:144-82-1 |
| Literature: WO2011/82400 A2, ; |
|
~% 
Sulfamethizole CAS#:144-82-1 |
| Literature: CH230860 , ; |
|
~% 
Sulfamethizole CAS#:144-82-1 |
| Literature: US2447702 , ; |
|
~% 
Sulfamethizole CAS#:144-82-1 |
| Literature: US2358031 , ; CH231190 , ; |
|
~% 
Sulfamethizole CAS#:144-82-1 |
| Literature: DE840544 , ; DRP/DRBP Org.Chem. |
| Precursor 7 | |
|---|---|
| DownStream 4 | |
| HS Code | 2935009090 |
|---|---|
| Summary | 2935009090 other sulphonamides VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:6.5% General tariff:35.0% |
|
Cheminformatics analysis of assertions mined from literature that describe drug-induced liver injury in different species.
Chem. Res. Toxicol. 23 , 171-83, (2010) Drug-induced liver injury is one of the main causes of drug attrition. The ability to predict the liver effects of drug candidates from their chemical structures is critical to help guide experimental... |
|
|
Translating clinical findings into knowledge in drug safety evaluation--drug induced liver injury prediction system (DILIps).
J. Sci. Ind. Res. 65(10) , 808, (2006) Drug-induced liver injury (DILI) is a significant concern in drug development due to the poor concordance between preclinical and clinical findings of liver toxicity. We hypothesized that the DILI typ... |
|
|
Developing structure-activity relationships for the prediction of hepatotoxicity.
Chem. Res. Toxicol. 23 , 1215-22, (2010) Drug-induced liver injury is a major issue of concern and has led to the withdrawal of a significant number of marketed drugs. An understanding of structure-activity relationships (SARs) of chemicals ... |
| MFCD00053363 |
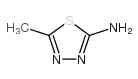
| 4-Amino-N-(5-methyl-1,3,4-thiadiazol-2-yl)benzenesulfonamide |
| Sulfamethizole |
| Sulfarine |
| EINECS 205-641-1 |
| Sulphamethizole |
| Benzenesulfonamide, 4-amino-N-(5-methyl-1,3,4-thiadiazol-2-yl)- |
![N-[4-[[(5-methyl-1,3,4-thiadiazol-2-yl)amino]sulphonyl]phenyl]acetamide structure](https://image.chemsrc.com/caspic/226/39719-87-4.png)

![Benzenesulfonamide,4-[2-(2,6-diamino-3-pyridinyl)diazenyl]-N-(5-methyl-1,3,4-thiadiazol-2-yl)- structure](https://image.chemsrc.com/caspic/087/29817-74-1.png) CAS#:29817-74-1
CAS#:29817-74-1![Benzenesulfonamide, 4-[2-(8-hydroxy-5-quinolinyl)diazenyl]-N-(5-methyl-1,3,4-thiadiazol-2-yl)- structure](https://image.chemsrc.com/caspic/368/29821-96-3.png) CAS#:29821-96-3
CAS#:29821-96-3![Benzenesulfonamide,4-[2-(4,5-dihydro-3-methyl-5-oxo-1-phenyl-1H-pyrazol-4-yl)diazenyl]-N-(5-methyl-1,3,4-thiadiazol-2-yl)- structure](https://image.chemsrc.com/caspic/057/29822-02-4.png) CAS#:29822-02-4
CAS#:29822-02-4
