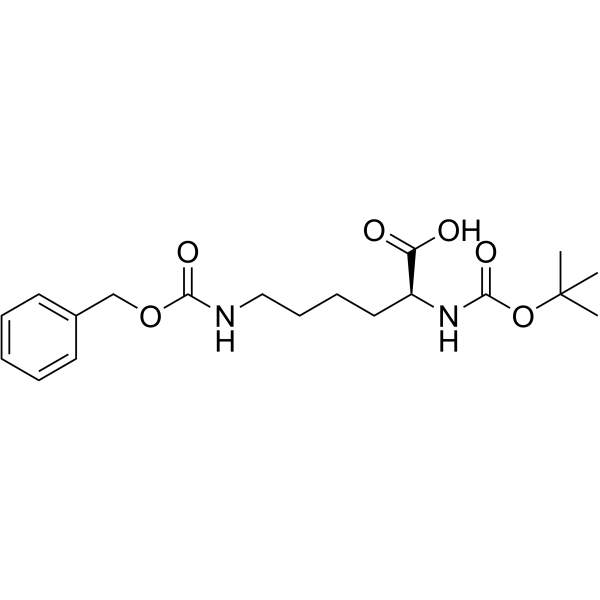
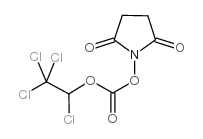
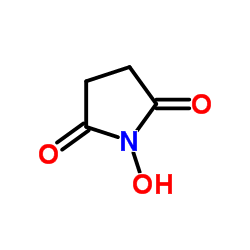
34404-36-9
| Name | Boc-Lys(Z)-OSu |
|---|---|
| Synonyms |
EINECS 251-999-7
2,5-Dioxopyrrolidin-1-yl N-[(benzyloxy)carbonyl]-N-(tert-butoxycarbonyl)-L-lysinate Boc-Lys-(CBz)-succinimide ester N2-[(1,1-Dimethylethoxy)carbonyl]-N6-[(phenylmethoxy)carbonyl]-L-lysine 2,5-dioxo-1-pyrrolidinyl ester NEPSILON-Benzyloxycarbonyl-NALPHA-tert-butoxycarbonyl-L-lysine-hydroxysuccinimid 2,5-Dioxo-1-pyrrolidinyl N-[(benzyloxy)carbonyl]-N-{[(2-methyl-2-propanyl)oxy]carbonyl}lysinate Boc-Lys(Ne-Cbz)-ONSu Lysine, N-[(1,1-dimethylethoxy)carbonyl]-N-[(phenylmethoxy)carbonyl]-, 2,5-dioxo-1-pyrrolidinyl ester MFCD00037915 NA-BOC-NE-CBZ-L-LYSINEHYDROXYSUCCINIMIDEESTER Benzyl (S)-(5-(((1,1-dimethylethoxy)carbonyl)amino)-6-((2,5-dioxo-1-pyrrolidinyl)oxy)-6-oxohexyl)carbamate N-(tert-butoxycarbonyl)-N-Z-L-lysine hydroxysuccinimide N-[(N2,N6-Dicarboxy-L-lysyl)oxy]succinimide N6-benzyl tert-butyl ester 2,5-dioxopyrrolidin-1-yl (S)-6-[(benzyloxycarbonyl)amino]-2-[(tert-butoxycarbonyl)amino]hexanoate L-Lysine, N-[(1,1-dimethylethoxy)carbonyl]-N-[(phenylmethoxy)carbonyl]-, 2,5-dioxo-1-pyrrolidinyl ester Boc-Lys(Z)-Osu Boc-Lyz(Z)-OSu |
| Description | Boc-Lys(Z)-OSu is a lysine derivative[1]. |
|---|---|
| Related Catalog | |
| In Vitro | Amino acids and amino acid derivatives have been commercially used as ergogenic supplements. They influence the secretion of anabolic hormones, supply of fuel during exercise, mental performance during stress related tasks and prevent exercise induced muscle damage. They are recognized to be beneficial as ergogenic dietary substances[1]. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Melting Point | 108-115ºC |
| Molecular Formula | C23H31N3O8 |
| Molecular Weight | 477.508 |
| Exact Mass | 477.211121 |
| PSA | 140.34000 |
| LogP | 1.62 |
| Index of Refraction | 1.557 |
| Storage condition | -20°C |
| Hazard Codes | Xi |
|---|---|
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
|
~83% 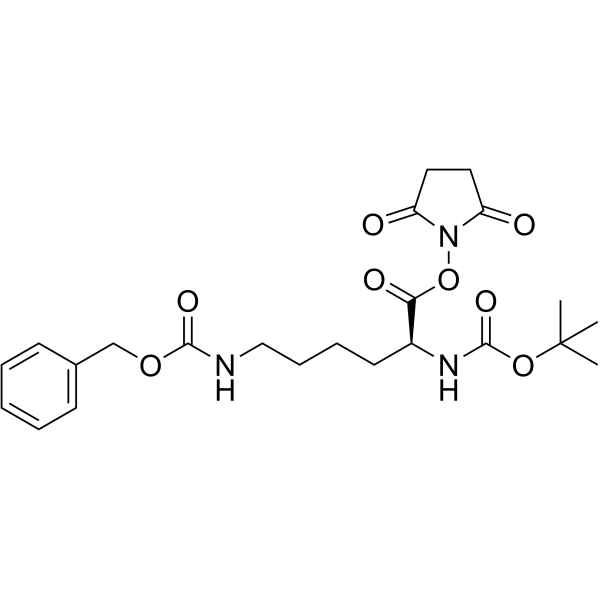
34404-36-9 |
| Literature: Jaoudai, Mahmoud; Martinez, Jean; Castro, Bertrand Journal of Organic Chemistry, 1987 , vol. 52, # 12 p. 2364 - 2367 |
|
~99% 
34404-36-9 |
| Literature: Rosowsky, Andre; Wright, Joel E. Journal of Organic Chemistry, 1989 , vol. 54, # 23 p. 5551 - 5558 |
| Precursor 3 | |
|---|---|
| DownStream 0 | |