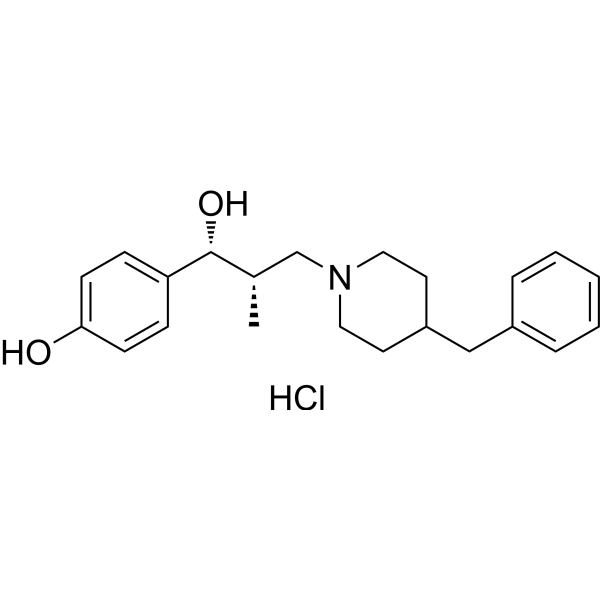
919289-58-0
| Name | 4-[(1R,2S)-3-(4-Benzyl-1-piperidinyl)-1-hydroxy-2-methylpropyl]ph enol hydrochloride (1:1) |
|---|
| Description | Ro 25-6981 hydrochloride is a potent, selective and activity-dependent NR2B subunit specific NMDA receptor antagonist. Ro 25-6981 hydrochloride shows anticonvulsant and anti-parkinsonian activity. Ro 25-6981 hydrochloride has the potential for the research of parkinson's disease (PD)[1][2][3]. |
|---|---|
| Related Catalog | |
| In Vitro | Ro 25-6981 Hydrochloride (0.39-12.5 mg/kg; i.p.) induces contraversive rotations in 6-hydroxydopamine (6-OHDA)-lesioned rats without stimulating locomotion in normal rats[1]. Ro 25-6981 Hydrochloride (1,3 mg/kg; i.p.) exhibits age- and activation-dependent anticonvulsant action at early postnatal development in rats[2]. Ro 25-6981 Hydrochloride (800 µg; intrathecal injection) shows significant analgesic effects on incision pain in rats and effectively attenuated postoperative hyperalgesia induced by remifentanil[3]. |
| In Vivo | Ro 25-6981 Hydrochloride (0.39-12.5 mg/kg; i.p.) induces contraversive rotations in 6-hydroxydopamine (6-OHDA)-lesioned rats without stimulating locomotion in normal rats[1]. Ro 25-6981 Hydrochloride (1,3 mg/kg; i.p.) exhibits age- and activation-dependent anticonvulsant action at early postnatal development in rats[2]. Ro 25-6981 Hydrochloride (800 µg; intrathecal injection) shows significant analgesic effects on incision pain in rats and effectively attenuated postoperative hyperalgesia induced by remifentanil[3]. Animal Model: 6-OHDA-lesioned rats[1] Dosage: 0.39-12.5 mg/kg Administration: I.p. Result: Dose-dependently induced contraversive tight nose-to-tail rotations, and induced a weak ipsiversive circling response indicating a mild unspecific stimulatory action of the compound. Animal Model: Male albino rats of Wistar strain[2] Dosage: 1, 3 mg/kg Administration: I.p. Result: Caused a significant decrease of N1–P2 amplitude at higher stimulation intensities AT 3 mg/kg, and exhibited age- and activation-dependent anticonvulsant action at early postnatal development. |
| References |
| Molecular Formula | C22H30ClNO2 |
|---|---|
| Molecular Weight | 375.93 |
| Exact Mass | 375.19700 |
| PSA | 43.70000 |
| LogP | 4.75630 |
| Hazard Codes | Xi |
|---|