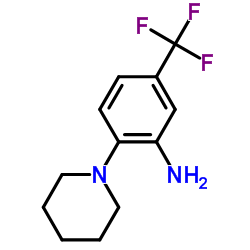
218156-96-8
| Name | N-[2-(1-Piperidinyl)-5-(trifluoromethyl)phenyl]isonicotinamide |
|---|---|
| Synonyms |
3,4-Dipropoxy-3-cyclobuten-1,2-dion
SR protein phosphorylation inhibitor 1 4-Pyridinecarboxamide (N-[2-(1-piperidinyl)-5-(trifluoromethyl)phenyl] squaric acid dibutyl ester N-[2-(1-Piperidinyl)-5-(trifluoromethyl)phenyl]isonicotinamide 1,2-di-n-propoxy-cyclobutenedione di-n-propyl squarate (SRPIN)340 N-[2-(1-Piperidinyl)-5-(trifluoromethyl)phenyl]-4-pyridinecarboxamide N4-[2-piperidino-5-(trifluoromethyl)phenyl]isonicotinamide 3-Cyclobutene-1,2-dione,3,4-dipropoxy SRPIN-340 SRPIN340 |
| Description | SRPIN340 is an ATP-competitive serine-arginine-rich protein kinase (SRPK) inhibitor, with a Ki of 0.89 μM for SRPK1. |
|---|---|
| Related Catalog | |
| Target |
Ki: 0.89 μM (SRPK1)[1] |
| In Vitro | SRPIN340 is a serine-arginine-rich protein kinase (SRPK) inhibitor, with a Ki of 0.89 μM for SRPK1. SRPIN340 also inhibits SRPK2, but shows no significant inhibition on other SRPK, such as Clk1 and Clk4. SRPIN340 promotes degradation of SRp75, which is necessary for HIV expression. SRPIN340 suppresses the propagation of Sindbis virus (IC50, 60 μM) as well as severe acute respiratory syndrome virus[1]. SRPIN340 shows inhibitory effect on leukemia cell lines, such as AML HL60, ALL-T Molt4 and Jurkat, with IC50s of 44.7 μM, 92.2 μM and 82.3 μM, respectively[2]. |
| Cell Assay | Leukemic cells (5 × 104 cells/well) and isolated PBMCs (8 × 104 cells/well) are seeded in 96-well plates. Each well contained 100 μL of complete RPMI medium and 100 μL of SRPIN340 solution at different concentrations. The compound is diluted in RPMI medium with 10% fetal bovine serum and 0.4% DMSO (v/v). After 48 h of culture, MTT (5 mg/mL) is added to the wells (3 h, 37°C). The plates are centrifuged at room temperature for 30 min 500 ×g, followed by the removal of the MTT solution and the addition of 100 μL/well of DMSO to solubilize the formazan. Absorbance is measured at 540 nm in a microplate reader. Each experimental procedure is performed in triplicate[2]. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 395.9±42.0 °C at 760 mmHg |
| Molecular Formula | C18H18F3N3O |
| Molecular Weight | 349.350 |
| Flash Point | 193.3±27.9 °C |
| Exact Mass | 349.140198 |
| PSA | 45.23000 |
| LogP | 4.15 |
| Appearance | light yellow solid |
| Vapour Pressure | 0.0±0.9 mmHg at 25°C |
| Index of Refraction | 1.578 |
| Storage condition | -20℃ |
|
~85% 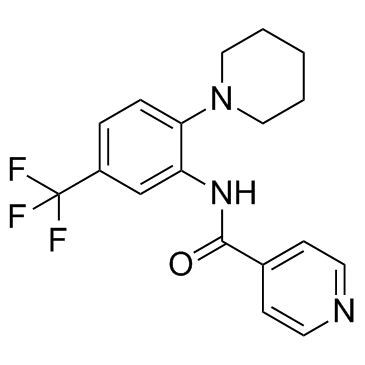
218156-96-8 |
| Literature: HAGIWARA, Masatoshi Patent: EP1712242 A1, 2006 ; Location in patent: Page/Page column 18; 31 ; |
| Precursor 2 | |
|---|---|
| DownStream 0 | |