885499-61-6
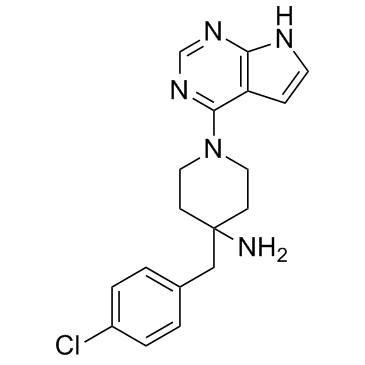
| Name | 4-[(4-chlorophenyl)methyl]-1-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)piperidin-4-amine |
|---|---|
| Synonyms |
M05
4-(4-Chlorobenzyl)-1-(7h-Pyrrolo[2,3-D]pyrimidin-4-Yl)piperidin-4-Aminium 4-[(4-chlorophenyl)methyl]-1-{7H-pyrrolo[2,3-d]pyrimidin-4-yl}piperidin-4-amine 4-(4-chlorobenzyl)-1-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)piperidin-4-amine 4-(4-Chlorobenzyl)-1-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-4-piperidinamine 4-(4-chlorobenzyl)-1-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)piperidin-4-ylamine CCT128930 |
| Description | CCT128930 is a potent and selective inhibitor of Akt2 (IC50 6 nM) with 28-fold selectivity over the closely related PKA kinase (IC50 168 nM), as well as 20-fold selectivity over p70S6K (IC50 120 nM). |
|---|---|
| Related Catalog | |
| Target |
Akt2:6 nM (IC50) p70S6K:120 nM (IC50) PKA:168 nM (IC50) Autophagy |
| In Vitro | CCT128930 exhibits marked antiproliferative activity and inhibits the phosphorylation of a range of Akt substrates in multiple tumor cell lines in vitro, consistent with Akt inhibition. CCT128930 causes a G1 arrest in PTEN-null U87MG human glioblastoma cells, consistent with Akt pathway blockade. CCT128930 is a potent ATP-competitive Akt inhibitor, which is initially screened at 10 µM against a panel of kinases representative of the human protein kinome. In view of the potential of ATP-competitive inhibitors to cross-react with the closely related AGC class of kinases, the IC50 of CCT128930 against selected AGC kinases is determined. The GI50 values of CCT128930 for growth inhibition are 6.3 μM±2.2 (n=3) for U87MG human glioblastoma cells, 0.35 μM±0.11 (n=4) for LNCaP human prostate cancer cells, and 1.9 μM±0.80 (n=5) for PC3 human prostate cancer cells, all of which are PTEN-deficient human tumor cell lines[1]. |
| In Vivo | The pharmacokinetics of CCT128930 after a single dose of 25 mg/kg are shown. Following i.v. administration, CCT128930 reaches a peak concentration of 6.4 µM in plasma and is eliminated with a relatively short half-life, high volume of distribution and rapid clearance, giving an AUC0-∞ of 4.6 µMh. Following i.p. administration, the peak plasma drug concentration is 4-fold lower and the plasma clearance is similar to that observed i.v..The corresponding AUC0-∞ is 1.3 µMh, giving an i.p. bioavailability of 29%[1]. |
| Kinase Assay | Profiling against 50 different human kinases is carried out using 10 μM CCT128930 at an ATP concentration equivalent to the Km for each enzyme. All other enzyme assays are performed[1]. |
| Cell Assay | All cell lines are grown in their recommended culture medium, supplemented with 10% fetal bovine serum at 37°C in 5% CO2 and passaged for less than six months prior to replacement from early passage frozen stocks. CCT128930 and LY294002 are made up as 10mM stocks in DMSO. Cells are regularly screened for Mycoplasma using a PCR-based assay. Cells are seeded in 96-well plates and allowed to attach for 36 hours to ensure exponential growth prior to treatment. In vitro antiproliferative activity is determined using a 96-hour SRB assay, and GI50 values are derived[1]. |
| Animal Admin | Mice[1] PTEN-null U87MG human glioblastoma cells (2×106) are injected subcutaneously (s.c.) in the right flank of 6-8 weeks old female CrTacNCr-Fox1nu mice. For HER2-positive, PIK3CA-mutant BT474 human breast cancer xenografts, cells (5×106) are administered s.c. in medium supplemented with matrigel (1:1) into the mammary fat pads of female mice implanted s.c. with estradiol pellets (0.025 mg, 90 day release) 3 days previously. Animals are randomized and treatment is started with vehicle or CCT128930 when established tumors are ~100 mm3 in mean volume. Control mice receive vehicle only (10% DMSO, 5% Tween 20, 85% saline) and treated mice received 50 mg/kg CCT128930 intraperitoneally (i.p.) daily for 5 days (U87MG human glioblastoma xenografts) or 40 mg/kg CCT128930 i.p. twice daily for 5 days (BT474 human breast cancer xenografts). Tumor size and body weight are monitored three times a week. Tumor size is evaluated by measurement of 2 orthogonal diameters with calipers and volume is calculated. At the end of the study, tumors are excised and weighed. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 547.9±50.0 °C at 760 mmHg |
| Molecular Formula | C18H20ClN5 |
| Molecular Weight | 341.838 |
| Flash Point | 285.2±30.1 °C |
| Exact Mass | 341.140717 |
| PSA | 70.83000 |
| LogP | 2.93 |
| Vapour Pressure | 0.0±1.5 mmHg at 25°C |
| Index of Refraction | 1.680 |
| Storage condition | -20℃ |