1439399-58-2
| Name | CB-839 |
|---|---|
| Synonyms |
CB839
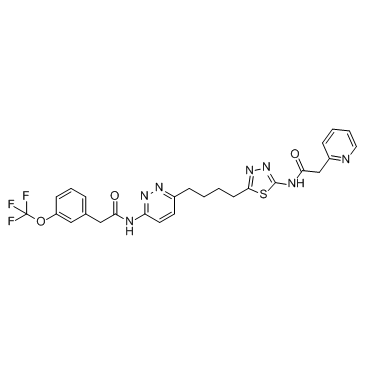
CB 839 2-(pyridin-2-yl)-N-(5-(4-(6-(2-(3-(trifluoromethoxy)phenyl)acetamido)pyridazin-3-yl)butyl)-1,3,4-thiadiazol-2-yl)acetamide 2-(2-Pyridinyl)-N-(5-{4-[6-({[3-(trifluoromethoxy)phenyl]acetyl}amino)-3-pyridazinyl]butyl}-1,3,4-thiadiazol-2-yl)acetamide Telaglenastat |
| Description | Telaglenastat (CB-839) is a potent and selective inhibitor of glutaminase with an IC50 of less than 50 nM. |
|---|---|
| Related Catalog | |
| Target |
IC50: 23 nM (glutaminase in kindey), 28 nM (glutaminase in brain)[1] |
| In Vitro | Telaglenastat has increased potency and distinct kinetic behavior, exhibiting a slow-on/slow-off mechanism. Telaglenastat has a potent effect on the proliferation of HCC1806 and MDA-MB-231 cell lines (IC50 of 20-55 nM associated with cell loss at >100 nM). TNBC cell lines are sensitive to glutaminase inhibition withTelaglenastat[1]. |
| In Vivo | Telaglenastat (200 mg/kg, p.o.) has antitumor activity in xenograft models of TNBC and basal-like breast cancer, and inhibits tumor glutaminase activity and changes metabolite levels[1]. |
| Cell Assay | For viability assays, all cell lines are treated with Telaglenastat at the indicated concentrations for 72 hours in duplicate wells and analyzed for antiproliferative effects using Cell Titer Glo. For all cell lines except MDA-MB-175, SUM149PT, Hs343.T, HCC38, and BT20 the results present represent an average across at least two independent experiments. IC50 values are calculated using a four parameter curve fit. Relative cell loss or proliferation in the presence of 1 μM Telaglenasta or in glutamine-free media is determined by comparing the CTG signals. |
| Animal Admin | Tumor growth studies are done in two xenograft models: (i) a patient-derived TNBC model, where tumor fragments isolated from the breast tissue of a 53-year-old Caucasian woman with stage IIa infiltrating ductal carcinoma are implanted subcutaneously into female nu/nu mice (age 4-6 weeks, 19-26 g) and (ii) a cell line model, where JIMT-1 cells are implanted subcutaneously at 1×107cells per mouse in the flank of female CB.17 SCID mice (age 8-12 weeks, 17-23 g). In both models, when tumors reach approximately 100-150 mm3, mice are dosed with vehicle or 200 mg/kg Telaglenastat (n=10/group) prepared in vehicle orally twice daily every 12 hours for 28 to 35 days. For the JIMT-1 model, two additional cohorts are dosed with paclitaxel prepared in 5% ethanol/5% Cremophor EL given as an intravenous bolus at 10 mg/kg every other day for 5 doses alone or in combination with 200 mg/kg CB-839 dosed orally twice daily. Tumor volumes and body weights are measured twice weekly. |
| References |
| Density | 1.430±0.06 g/cm3 |
|---|---|
| Molecular Formula | C26H24F3N7O3S |
| Molecular Weight | 571.574 |
| Exact Mass | 571.161316 |
| PSA | 160.12000 |
| LogP | 2.61 |
| Appearance | Yellow solid |
| Index of Refraction | 1.635 |
| Storage condition | -20℃ |
| Water Solubility | Insuluble (8.0E-4 g/L) (25 ºC) |