87760-53-0
| Name | Tandospirone |
|---|---|
| Synonyms |
Tandospirone
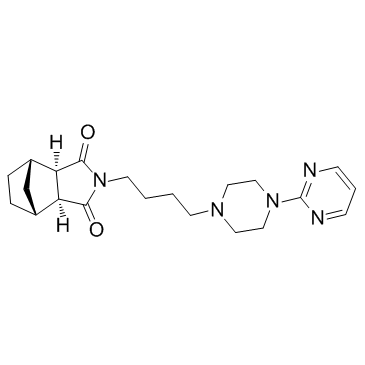
(3aR,4S,7R,7aS)-2-{4-[4-(pyrimidin-2-yl)piperazin-1-yl]butyl}hexahydro-1H-4,7-methanoisoindole-1,3(2H)-dione metanopirone [14C]-Tandospirone (1R*,2S*,3R*,4S*)-N-[4-[4-(2-pyrimidinyl)-1-piperazinyl]butyl]-2,3-bicyclo[2.2.1]heptanedicarboximide (1R,2S,6R,7S)-4-{4-[4-(2-Pyrimidinyl)-1-piperazinyl]butyl}-4-azatricyclo[5.2.1.0]decane-3,5-dione (1R,2S,6R,7S)-4-{4-[4-(Pyrimidin-2-yl)piperazin-1-yl]butyl}-4-azatricyclo[5.2.1.0]decane-3,5-dione (3aa,4b,7b,7aa)-Hexahydro-2-[4-[4-(2-pyrimidinyl)-1-piperazinyl]butyl]-4,7-methano-1H-isoindole-1,3(2H)-dione Sediel |
| Description | Tandospirone(SM-3997) is a potent and selective 5-HT1A receptor partial agonist (Ki = 27 nM) that displays selectivity over SR-2, SR-1C, α1, α2, D1 and D2 receptors (Ki values ranging from 1300-41000 nM). IC50 Value: 27±5 nM(Ki) [1]Target: 5-HT1Ain vitro: Tandospirone is most potent at the 5-HT1A receptor, displaying a Ki value of 27 +/- 5 nM. The agent is approximately two to three orders of magnitude less potent at 5-HT2, 5-HT1C, alpha 1-adrenergic, alpha 2-adrenergic, and dopamine D1 and D2 receptors (Ki values ranging from 1300 to 41000 nM). Tandospirone is essentially inactive at 5-HT1B receptors; 5-HT uptake sites; beta-adrenergic, muscarinic cholinergic, and benzodiazepine receptors [1]. 3H-SM-3997 bound rapidly, reversibly and in a saturable manner with high affinity to rat brain hippocampal membranes (Kd = 9.4 nM, Bmax = 213 fmol/mg protein) [2]. in vivo: Chronic treatment with tandospirone, at 0.2 and 1.0mg/kg/day, but not 2.0mg/kg/day, attenuated footshock stress-induced eLAC elevation in the mPFC [3]. Rats were acutely administered tandospirone (0, 0.1, and 1 mg/kg, i.p.). Tandospirone decreased the number of premature responses, an index of impulsive action, in a dose-dependent manner [4].Toxicity: It is not believed to be addictive but it is known to produce mild withdrawal effects (e.g. anorexia) after abrupt discontinuation. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 613.9±65.0 °C at 760 mmHg |
| Melting Point | 112-113.5° |
| Molecular Formula | C21H29N5O2 |
| Molecular Weight | 383.487 |
| Flash Point | 325.1±34.3 °C |
| Exact Mass | 383.232117 |
| PSA | 69.64000 |
| LogP | 2.02 |
| Vapour Pressure | 0.0±1.8 mmHg at 25°C |
| Index of Refraction | 1.589 |
| Storage condition | Store at +4°C |
| Water Solubility | DMSO: soluble38mg/mL |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Hazard Codes | Xi |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26 |
| RIDADR | NONH for all modes of transport |
| Precursor 8 | |
|---|---|
| DownStream 0 | |
![(1R*,2S*,3R*,4S*)-N-[4-[4-(2-Pyrimidinyl)-1-piperazinyl]-2-butynyl]-2,3-bicyclo[2.2.1]heptanedicarboximide structure](https://image.chemsrc.com/caspic/075/120596-77-2.png)

![8-(2-Pyrimidinyl)-8-aza-5-azoniaspiro[4.5]decane Bromide structure](https://image.chemsrc.com/caspic/372/81461-73-6.png)

