75614-89-0
| Name | R(-)-α-Methyl Histamine Dihydrochloride |
|---|---|
| Synonyms | R(-)-α-Methylhistamine Dihydrochloride |
| Description | (R)-(-)-α-Methylhistamine dihydrochloride is a potent, selective and brain-penetrant agonist of H3 histamine receptor, with a Kd of 50.3 nM[1][2]. (R)-(-)-α-Methylhistamine dihydrochloride can enhance memory retention, attenuates memory impairment in rats[3][4][5]. |
|---|---|
| Related Catalog | |
| Target |
H3 Receptor:50.3 nM (Kd) |
| In Vitro | (R)-(-)-α-Methylhistamine dihydrochloride is an H3-agonist that is >10 times as potent as histamine (HA). Its selectivity toward H3-receptors is >1000 times as high as that of HA. (R)-(-)-α-Methylhistamine dihydrochloride has only weak affinities for H1 and H2 receptor with a pKi=4.8 and <3.5, repectively. (R)-(-)-α-Methylhistamine dihydrochloride displays >200-fold selectivity over H4 receptors[1][2][3]. |
| In Vivo | Pretreatment with (R)-(-)-α-Methylhistamine dihydrochloride (RAMH; 10 mg/kg; i.p.; 60 min before training) reverses Propofol‐induced (25 mg/kg; i.p.; 30 min before training) memory retention[5]. (R)-(-)-α-Methylhistamine dihydrochloride (6.3 mg/kg; i.p.) significantly decreases the steady-state t-MH level in the mouse brain, whereas these compounds produced no significant changes in the HA level[3]. Animal Model: Male Sprague‐Dawley rats (10-12 week)[3] Dosage: 10 mg/kg Administration: IP; 60 min before training Result: Reversed propofol‐induced memory retention. |
| References |
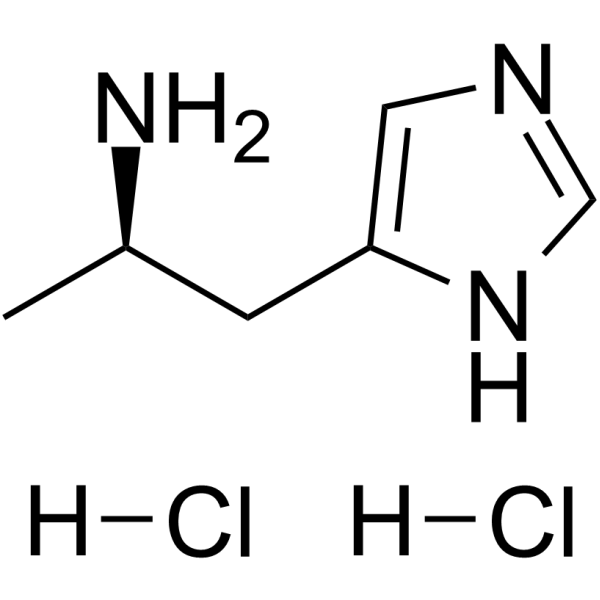
| Molecular Formula | C6H13Cl2N3 |
|---|---|
| Molecular Weight | 198.09400 |
| Exact Mass | 197.04900 |
| PSA | 54.70000 |
| LogP | 2.77620 |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi: Irritant; |
| Risk Phrases | 36/37/38 |
| Safety Phrases | 26-36 |
| RIDADR | NONH for all modes of transport |