50935-04-1
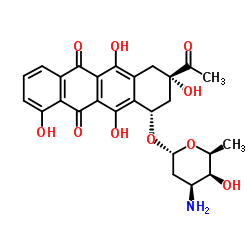
| Name | 5,12-Naphthacenedione, 8-acetyl-10-[[3-amino-2,3,6-trideoxy-.α.-L-lyxo-hexopyranosyl]oxy]-7,8,9,10-tetrahydro-1,6,8,11-tetrahydroxy-, (8S-cis) |
|---|---|
| Synonyms |
(1S,3S)-3-Acetyl-3,5,10,12-tetrahydroxy-6,11-dioxo-1,2,3,4,6,11-hexahydro-1-tetracenyl 3-amino-2,3,6-trideoxy-α-L-lyxo-hexopyranoside
Carminomycin (1S,3S)-3-Acetyl-3,5,10,12-tetrahydroxy-6,11-dioxo-1,2,3,4,6,11-hexahydrotetracen-1-yl 3-amino-2,3,6-trideoxy-α-L-lyxo-hexopyranoside Carminomycin I Antibiotic R-588A Carminomicin I (1S,3S)-3-Acetyl-1,2,3,4,6,11-hexahydro-3,5,10,12-tetrahydroxy-6,11-dioxo-1-naphthacenyl 3-Amino-2,3,6-trideoxy-a-L-lyxo-hexopyranoside Karminomitsin 4-O-Demethyldaunorubicin Karminomycin (8S-cis)-Acetyl-10-[(3-amino-2,3,6-trideoxy-a-L-lyxo-hexopyranosyl)oxy]-7,8,9,10-tetrahydro-1,6,8,11-tetrahydroxy-5,12-naphthacenedione Carubicin |
| Description | Carubicin (Carminomycin) is a microbially-derived compound. Carubicin is an effective inhibitor of VHL-defective (VHL−/−) CCRCC cell proliferation. Carubicin also induces apoptosis by a mechanism independent of p53 or hypoxia-inducible factor HIF2. Carubicin has the potential for the research of cancer diseases[1][2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Boiling Point | 750.6±60.0 °C at 760 mmHg |
| Molecular Formula | C26H27NO10 |
| Molecular Weight | 513.493 |
| Flash Point | 407.7±32.9 °C |
| Exact Mass | 513.163513 |
| PSA | 196.84000 |
| LogP | 3.54 |
| Vapour Pressure | 0.0±2.6 mmHg at 25°C |
| Index of Refraction | 1.727 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
|
~% 
50935-04-1 |
| Literature: Zabudkin, Alexander F.; Matvienko, Victor; Matveev, Alexey; Itkin, Aleksandr M. Patent: US2007/135624 A1, 2007 ; Location in patent: Page/Page column 2 ; |
| Precursor 1 | |
|---|---|
| DownStream 0 | |
