15687-41-9
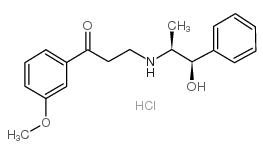
| Name | Oxyfedrine |
|---|---|
| Synonyms |
3-[[(1R,2S)-2-Hydroxy-1-methyl-2-phenylethyl]amino]-1-(3-methoxyphenyl)-1-propanone
(r-(r*,s*))-3-((2-hydroxy-1-methyl-2-phenylethyl)amino)-1-( 3-methoxyphenyl)-1-propanone 3-{[(1S,2R)-2-Hydroxy-1-methyl-2-phenylethyl]amino}-1-[3-(methyloxy)phenyl]propan-1-one |
| Description | Oxyfedrine, a vasodilator, is an orally active β-adrenoreceptor agonist. Oxyfedrine decreases the tonicity of coronary vessels. Oxyfedrine can be used in the research of cardiovascular disease[1][2]. |
|---|---|
| Related Catalog | |
| Target |
β-adrenoceptor |
| In Vitro | Oxyfedrine (50 μM, 48 h) suppresses aldehyde dehydrogenase (ALDH) activity in HCT116 and HSC-4 cells[1]. Oxyfedrine (50 μM, 48 h) acts as a sensitizer for GSH-depleting agents, and induces cell death in HCT116 and HSC-4 cells when with the drug combinations[1]. Oxyfedrine (0-1 μg/mL) inhibits spontaneous myogenic activity in rat isolated portal vein[4]. |
| In Vivo | Oxyfedrine (14 mg/kg, p.o., for 3-4 weeks) shows anti-anginal action in cats[2]. Oxyfedrine (10 mg/kg, i.p., HCT116 cell xenograft mice) suppresses tumor growth when combined with sulfasalazine (SSZ, 350 mg/kg, i.p.)[1]. Oxyfedrine (1 mg/kg, i.v.) decreases the arterial and venous blood high blood viscosity (HBV) in ice water stress rats[3]. Animal Model: Cats[2] Dosage: 14 mg/kg Administration: Oral administration (p.o.), for 3-4 weeks. Result: Decreased systolic and diastolic blood pressures, increased heart rate and cardiac output. |
| Density | 1.122 g/cm3 |
|---|---|
| Boiling Point | 475.2ºC at 760 mmHg |
| Molecular Formula | C19H24ClNO3 |
| Molecular Weight | 349.85200 |
| Flash Point | 241.2ºC |
| Exact Mass | 349.14400 |
| PSA | 58.56000 |
| LogP | 4.17260 |
| Vapour Pressure | 7.78E-10mmHg at 25°C |
| Index of Refraction | 1.566 |