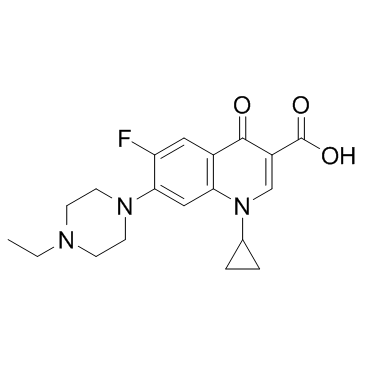
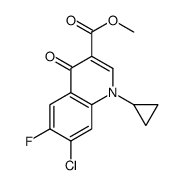
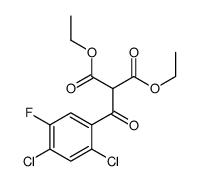
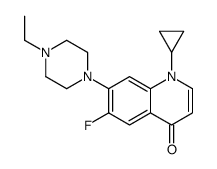
93106-60-6
| Name | enrofloxacin |
|---|---|
| Synonyms |
bayvp2674
Enorfloxacin Enrolfoxacin 1-cyclopropyl-7-(4-ethyl-piperazinyl)-6-fluoro-1,4-dihydro-4-oxo-quinoline-3-carboxylic acid cfpq Enrofoxacin Enrofloxacin 1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-7-(4-ethyl-1-piperazinyl)-quinoline-3-carboxylic acid 1-cyclopropyl-7-(4-ethyl-1-piperazinyl)-6-fluoro-1,4-dihydro-4-oxo-3-quinolinecarboxylic acid ROFLOXACIN BASE MFCD01861684 Baytri 1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-7-(4-ethyl-1-piperazinyl)-3-quinolinecarboxylic acid 1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-7-(4-ethylpiperazino)-quinoline-3-carboxylic acid |
| Description | Enrofloxacin is an effective antibiotic with an MIC90 of 0.312 μg/mL for Mycoplasma bovis. |
|---|---|
| Related Catalog | |
| Target |
MIC90: 0.312 μg/mL ( Mycoplasma bovis)[1] |
| In Vitro | Mycoplasma bovis is a worldwide pathogen, causative agent of pneumonia, mastitis, arthritis, and a variety of other symptoms in cattle. The antibiotic susceptibility profiles of the Hungarian strains are consistent within the tested group of fluoroquinolones. Three isolates (MYC44, MYC45 and MYC46) have high MIC values (≥10 μg/mL) to Enrofloxacin, while the rest of the strains are inhibited by Enrofloxacin with MICs ≤0.312 or 0.625 μg/mL[1]. |
| In Vivo | Mice (n=80) undergo transient middle cerebral artery occlusion (MCAo) with reperfusion after 60 minutes. After MCAo, animals are randomly assigned to receive either a daily preventive medication (n=26, Enrofloxacin) starting at the day of MCAo or a therapeutic medication (n=25; Enrofloxacin) after diagnosis of lung infection. Standard treatment started immediately after the appearance of clinical signs (general health score>6) usually between day 4 and 6 after stroke. Both, preventive and standard antibiotic treatment using Enrofloxacin improve survival in a similar way compared with placebo treatment[2]. |
| Animal Admin | Mice[2] 11- to 14-week-old C57Bl6/J male mice are used. Enrofloxacin (2.5% oral solution) is dispensed in saline (2 mg/mL), antibiotic-treated animals receive a daily orally dispensed dose of 10 mg/kg body weight via feeding needle every 12 hours over a period of 7 days, while placebo animals receive the same amount of saline via feeding needle. Animals of preventive antibiotic group obtained Enrofloxacin after waking from reperfusion anesthesia (ca. 1 hour after operation). Therapeutic antibiotic treatment is given immediately after appearance of clinical signs (general health score>5) and confirmation of lung infection by MRI (signal rate≥5%). The group allocation is randomized[2]. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 560.5±50.0 °C at 760 mmHg |
| Melting Point | 225 °C |
| Molecular Formula | C19H22FN3O3 |
| Molecular Weight | 359.395 |
| Flash Point | 292.8±30.1 °C |
| Exact Mass | 359.164520 |
| PSA | 65.78000 |
| LogP | 1.88 |
| Vapour Pressure | 0.0±1.6 mmHg at 25°C |
| Index of Refraction | 1.634 |
| Storage condition | -20°C Freezer |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xi: Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | 26-36/37 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | VB1993650 |
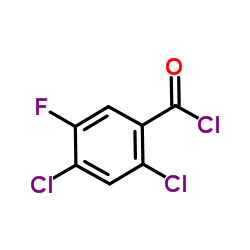
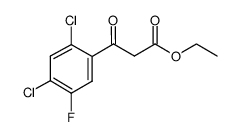
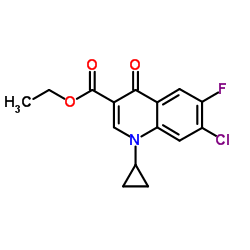
| Precursor 10 | |
|---|---|
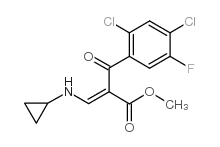
| DownStream 1 | |