70563-58-5
| Name | herbimycin |
|---|---|
| Synonyms |
herbimycin
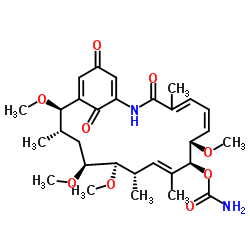
MFCD00151702 Herbimycin A (4E,6Z,8S,9S,10E,12S,13R,14S,16S,17R)-9-[(aminocarbonyl)oxy]-8,13,14,17-tetramethoxy-4,10,12,16-tetramethyl-2-azabicyclo[16.3.1]docosa-4,6,10,18,21-pentaene-3,20,22-trione (15R)-17-demethoxy-15-methoxy-11-O-methylgeldanamycin (4E,6Z,8S,9S,10E,12S,13R,14S,16S,17R)-8,13,14,17-Tetramethoxy-4,10,12,16-tetramethyl-3,20,22-trioxo-2-azabicyclo[16.3.1]docosa-1(21),4,6,10,18-pentaen-9-yl carbamate |
| Description | Herbimycin A, an ansamycin antibiotic, acts as a Src family kinase inhibitor. Herbimycin A binds to the SH domain and inhibits the activity of p60v-src and p210BCR-ABL. Herbimycin A inhibits Hsp90 and impairs recovery from heat shock. Herbimycin A exhibits antiangiogenic activity in endothelial cells in vitro. |
|---|---|
| Related Catalog |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 752.4±60.0 °C at 760 mmHg |
| Molecular Formula | C30H42N2O9 |
| Molecular Weight | 574.662 |
| Flash Point | 408.8±32.9 °C |
| Exact Mass | 574.289001 |
| PSA | 152.48000 |
| LogP | 2.12 |
| Vapour Pressure | 0.0±2.5 mmHg at 25°C |
| Index of Refraction | 1.545 |
| Storage condition | −20°C |
| Water Solubility | DMSO: 7.5 mg/mL DMSO stock solution can be diluted in phosphate buffered saline. The ratio of buffer:DMSO should be greater than 500:1. |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xi |
| Risk Phrases | R36/37/38 |
| Safety Phrases | 22-24/25-36-26 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | LX8930000 |
| HS Code | 29419000 |
| Precursor 1 | |
|---|---|
| DownStream 0 | |
| HS Code | 29419000 |
|---|