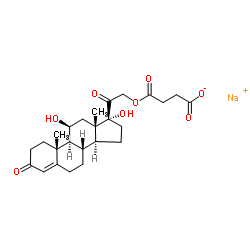
125-04-2
| Name | Hydrocortisone Hemisuccinate Sodium Salt |
|---|---|
| Synonyms |
Sodium 4-{[(11β)-11,17-dihydroxy-3,20-dioxopregn-4-en-21-yl]oxy}-4-oxobutanoate
Solu-Glyc Butanedioic acid, mono[(11β)-11,17-dihydroxy-3,20-dioxopregn-4-en-21-yl] ester, sodium salt (1:1) Hydrocortisone hemisuccinate sodium salt Natrium-4-{2-[(8S,9S,10R,11S,13S,14S,17R)-11,17-dihydroxy-10,13-dimethyl-3-oxo-2,3,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl]-2-oxoethoxy}-4-oxobutanoat Intracort Hydrocortisone sodium succinate MFCD00069472 (11b)-21-(3-Carboxy-1-oxopropoxy)-11,17-dihydroxypregn-4-ene-3,20-dione Monosodium Salt Saxizon EINECS 204-725-5 Buccalsone butanedioic acid, mono[(11β)-11,17-dihydroxy-3,20-dioxopregn-4-en-21-yl] ester, monosodium salt Hydrocortisone 21-sodium succinate Corlan sodium 4-{2-[(8S,9S,10R,11S,13S,14S,17R)-11,17-dihydroxy-10,13-dimethyl-3-oxo-2,3,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl]-2-oxoethoxy}-4-oxobutanoate Nordicort 4-{2-[(8S,9S,10R,11S,13S,14S,17R)-11,17-dihydroxy-10,13-diméthyl-3-oxo-2,3,6,7,8,9,10,11,12,13,14,15,16,17-tétradécahydro-1H-cyclopenta[a]phénanthrén-17-yl]-2-oxoéthoxy}-4-oxobutanoate de sodium Sodium 17-hydroxycorticosterone 21-succinate |
| Description | Hydrocortisone hemisuccinate sodium is an orally active physiological glucocorticoid. Hydrocortisone hemisuccinate sodium inhibits proinflammatory cytokine activity, with IC50s of 6.7 and 21.4 μM for IL-6 and IL-3, respectively. Hydrocortisone hemisuccinate sodium can be used for the research of ulcerative colitis (UC)[1]. |
|---|---|
| Related Catalog | |
| In Vitro | Hydrocortisone hemisuccinate sodium inhibits IL-6 and IL-3 bioactivity, with IC50s of 6.7 and 21.4 μM, respectively, and shows no cytotoxic effects on IL-6-independent MH60 cells[3]. Hydrocortisone hemisuccinate sodium (0.12-60 μM; 72 h) inhibits phytohemagglutinin (PHA) response in peripheral lymphocytes (PBL) and T-lymphocytes cultures[3]. |
| In Vivo | Hydrocortisone hemisuccinate sodium (30 mg/kg; p.o. twice daily for 5 d) reduces the weight loss and increases the food intake in mice[2]. Animal Model: Male Sprague-Dawley rats (200-220 g, 10-11 weeks) are induced colitis[2] Dosage: 30 mg/kg Administration: P.o. twice daily for 5 days Result: Significantly decreased the disease activity index (DAI) scores and myeloperoxidase (MPO) activity compared to the 2, 4, 6-trinitrobenzenesulfonic acid (TNBS) group. Increased the body weight. |
| References |
| Boiling Point | 685.5ºC at 760mmHg |
|---|---|
| Molecular Formula | C25H33NaO8 |
| Molecular Weight | 484.51 |
| Flash Point | 231.1ºC |
| Exact Mass | 484.207306 |
| PSA | 141.03000 |
| LogP | 0.86260 |
| Appearance | powder |
| Vapour Pressure | 9.36E-22mmHg at 25°C |
| Storage condition | −20°C |
| Water Solubility | H2O: 50 mg/mL |
|
Section 1. Chemical Product and Company Identification Hydrocortisone Sodium SuccinateCatalog Common Name/ Number(s). Trade Name CAS#125-04-2 Manufacturer
Commercial Name(s) Hydrocortisone Sodium Succinate Section 3. Hazards Identification Potential Acute Health Effects Slightly hazardous in case of skin contact (irritant), of eye contact (irritant), of ingestion, of inhalation. Potential Chronic HealthCARCINOGENIC EFFECTS: Not available. EffectsMUTAGENIC EFFECTS: Not available. TERATOGENIC EFFECTS: Not available. DEVELOPMENTAL TOXICITY: Not available. Repeated or prolonged exposure is not known to aggravate medical condition. Section 4. First Aid Measures Eye ContactCheck for and remove any contact lenses. In case of contact, immediately flush eyes with plenty of water for at least 15 minutes. Cold water may be used. Get medical attention if irritation occurs. Skin ContactWash with soap and water. Cover the irritated skin with an emollient. Get medical attention if irritation develops. Cold water may be used. Serious Skin ContactNot available. InhalationIf inhaled, remove to fresh air. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical attention. Serious InhalationNot available. IngestionDo NOT induce vomiting unless directed to do so by medical personnel. Never give anything by mouth to an unconscious person. If large quantities of this material are swallowed, call a physician immediately. Loosen tight clothing such as a collar, tie, belt or waistband. Serious IngestionNot available. Section 5. Fire and Explosion Data Flammability of the Product May be combustible at high temperature. Auto-Ignition Temperature Not available. Flash PointsNot available. Flammable LimitsNot available. These products are carbon oxides (CO, CO2). Some metallic oxides. Products of Combustion Fire Hazards in Presence of Slightly flammable to flammable in presence of heat. Various Substances Explosion Hazards in Presence Risks of explosion of the product in presence of mechanical impact: Not available. of Various SubstancesRisks of explosion of the product in presence of static discharge: Not available. Fire Fighting MediaSMALL FIRE: Use DRY chemical powder. and InstructionsLARGE FIRE: Use water spray, fog or foam. Do not use water jet. Special Remarks onAs with most organic solids, fire is possible at elevated temperatures. Fire HazardsMaterial in powder form, capable of creating a dust explosion. Special Remarks on Explosion Fine dust dispersed in air in sufficient concentrations, and in the presence of an ignition source is a potential dust Hazardsexplosion hazard. Hydrocortisone Sodium Succinate Section 6. Accidental Release Measures Small SpillUse appropriate tools to put the spilled solid in a convenient waste disposal container. Finish cleaning by spreading water on the contaminated surface and dispose of according to local and regional authority requirements. Large SpillUse a shovel to put the material into a convenient waste disposal container. Finish cleaning by spreading water on the contaminated surface and allow to evacuate through the sanitary system. Section 7. Handling and Storage PrecautionsKeep away from heat. Keep away from sources of ignition. Ground all equipment containing material. Do not breathe dust. Keep away from incompatibles such as oxidizing agents. StorageKeep container tightly closed. Keep container in a cool, well-ventilated area. Do not store above -20°C (-4°F). Freeze. Section 8. Exposure Controls/Personal Protection Engineering ControlsUse process enclosures, local exhaust ventilation, or other engineering controls to keep airborne levels below recommended exposure limits. If user operations generate dust, fume or mist, use ventilation to keep exposure to airborne contaminants below the exposure limit. Personal ProtectionSafety glasses. Lab coat. Dust respirator. Be sure to use an approved/certified respirator or equivalent. Gloves. Personal Protection in Case of Splash goggles. Full suit. Dust respirator. Boots. Gloves. A self contained breathing apparatus should be used a Large Spillto avoid inhalation of the product. Suggested protective clothing might not be sufficient; consult a specialist BEFORE handling this product. Exposure LimitsNot available. Section 9. Physical and Chemical Properties Physical state and appearance Solid. (Powdered solid.)OdorNot available. TasteNot available. Molecular Weight484.52 g/mole White. Off-white. Color Not available. pH (1% soln/water) Boiling PointNot available. Not available. Melting Point Critical TemperatureNot available. Specific GravityNot available. Vapor PressureNot applicable. Vapor DensityNot available. VolatilityNot available. Odor ThresholdNot available. Water/Oil Dist. Coeff.Not available. Ionicity (in Water)Not available. Dispersion PropertiesSee solubility in water. SolubilitySoluble in cold water. Hydrocortisone Sodium Succinate Section 10. Stability and Reactivity Data The product is stable. Stability Instability TemperatureNot available. Excess heat, incompatible materials, moisture Conditions of Instability Reactive with oxidizing agents. Incompatibility with various substances CorrosivityNot available. Special Remarks onNot available. Reactivity Special Remarks onNot available. Corrosivity PolymerizationWill not occur. Section 11. Toxicological Information Routes of EntryInhalation. Ingestion. Toxicity to AnimalsLD50: Not available. LC50: Not available. Chronic Effects on Humans Not available. Other Toxic Effects onSlightly hazardous in case of skin contact (irritant), of ingestion, of inhalation. Humans Special Remarks onNot available. Toxicity to Animals Special Remarks onMay cause adverse reproductive effects and birth defects (teratogenic) based on animal test data. Chronic Effects on Humans Special Remarks on otherAcute Potential Health Effects: Toxic Effects on HumansSkin: May cause skin irritation. Eyes: May cause eye irritation. Inhalation: May cause respiratory tract irritation. Ingestion: May cause nausea and vomiting. May affect respiration, cardiovascular system. Chronic Potential Health Effects: Ingestion: Prolonged or repeated ingestion may cause weight loss, nausea, vomiting. It may also affect the blood (anemia), adrenal gland. Section 12. Ecological Information EcotoxicityNot available. BOD5 and CODNot available. Possibly hazardous short term degradation products are not likely. However, long term degradation products may Products of Biodegradation arise. The product itself and its products of degradation are not toxic. Toxicity of the Products of Biodegradation Special Remarks on theNot available. Products of Biodegradation Hydrocortisone Sodium Succinate Section 13. Disposal Considerations Waste DisposalWaste must be disposed of in accordance with federal, state and local environmental control regulations. Section 14. Transport Information DOT ClassificationNot a DOT controlled material (United States). Not applicable. Identification Not applicable. Special Provisions for Transport DOT (Pictograms) Section 15. Other Regulatory Information and Pictograms No products were found. Federal and State Regulations CaliforniaCalifornia prop. 65: This product contains the following ingredients for which the State of California has found to cause cancer which would require a warning under the statute: No products were found. Proposition 65 Warnings California prop. 65: This product contains the following ingredients for which the State of California has found to cause birth defects which would require a warning under the statute: No products were found. Other RegulationsEINECS: This product is on the European Inventory of Existing Commercial Chemical Substances (EINECS No. 204-725-5). Canada: Listed on Canadian Domestic Substance List (DSL). China: Not listed on National Inventory. Japan: Listed on National Inventory (ENCS). Korea: Listed on National Inventory (KECI). Philippines: Not listed on National Inventory (PICCS). Australia: Listed on AICS. WHMIS (Canada) Not controlled under WHMIS (Canada). Other Classifications DSCL (EEC)This product is not classified according Not applicable. to the EU regulations. Health Hazard HMIS (U.S.A.)1 National Fire Protection 1 Flammability 1 Association (U.S.A.) Fire Hazard 1 0 Reactivity Health Reactivity 0 Specific hazard Personal Protection E WHMIS (Canada) (Pictograms) DSCL (Europe) (Pictograms) Hydrocortisone Sodium Succinate TDG (Canada) (Pictograms) ADR (Europe) (Pictograms) Protective Equipment Gloves. Lab coat. Dust respirator. Be sure to use an approved/certified respirator or equivalent. SECTION 16 - ADDITIONAL INFORMATION N/A |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xi |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | GM9015000 |