477575-56-7
| Name | PHA-665752 hydrate |
|---|---|
| Synonyms |
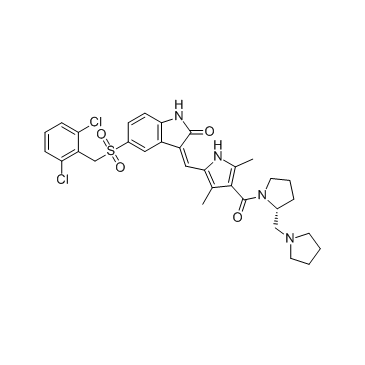
(3Z)-5-[(2,6-dichlorophenyl)methylsulfonyl]-3-[[3,5-dimethyl-4-[(2R)-2-(pyrrolidin-1-ylmethyl)pyrrolidine-1-carbonyl]-1H-pyrrol-2-yl]methylidene]-1H-indol-2-one
TCMDC-125885 (3Z)-5-[(2,6-Dichlorobenzyl)sulfonyl]-3-[(3,5-dimethyl-4-{[(2R)-2-(1-pyrrolidinylmethyl)-1-pyrrolidinyl]carbonyl}-1H-pyrrol-2-yl)methylene]-1,3-dihydro-2H-indol-2-one (3Z)-5-[(2,6-Dichlorobenzyl)sulfonyl]-3-[(3,5-dimethyl-4-{[(2R)-2-(pyrrolidin-1-ylmethyl)pyrrolidin-1-yl]carbonyl}-1H-pyrrol-2-yl)methylene]-1,3-dihydro-2H-indol-2-one PHA-665752 |
| Description | PHA-665752 is a potent, selective and ATP-competitive c-Met inhibitor with IC50 of 9 nM, >50-fold selectivity for c-Met than RTKs or STKs.IC50 value: 9 nMTarget: c-Metin vitro: PHA-665752 significantly inhibits c-Met kinase activity with Ki of 4 nM, and exhibits >50-fold selectivity for c-Met compared with various tyrosine and serine-threonine kinases. PHA-665752 potently inhibits the HGF-stimulated c-Met autophosphorylation with IC50 of 25-50 nM. PHA-665752 also significantly blocks HGF- and c-Met-dependent functions such as cell motility and cell proliferation with IC50 of 40-50 nM and 18-42 nM, respectively. In addition, PHA-665752 potently inhibits HGF-stimulated or constitutive phosphorylation of mediators of downstream of c-Met such as Gab-1, ERK, Akt, STAT3, PLC-γ, and FAK in multiple tumor cell lines [1]. PHA-665752 inhibits cell growth in TPR-MET-transformed BaF3 cells with IC50 of <60 nM, and inhibits constitutive cell motility and migration by 92.5% at 0.2 μM. Inhibition of c-Met by PHA665752 (0.2 μM) also induces cell apoptosis of 33.1% and G1 cell cycle arrest with cells in G1 phase increasing from 42.4% to 77.0%. PHA665752 can cooperate with rapamycin to inhibit cell growth of TPR-MET-transformed BaF3 cells and non-small cell lung cancer H441 cells [2].in vivo: Administration of PHA-665752 induces a dose-dependent tumor growth inhibition of S114 xenografts by 20 %, 39% and 68%, at dose of 7.5, 15, and 30 mg/kg/day, respectively [1]. PHA665752 treatment significantly reduces the tumor growth of NCI-H69, NCI-H441 and A549 in mouse xenografts by 99%, 75%, and 59%, respectively. PHA665752 also significantly inhibits angiogenesis by >85%, due to decreasing the production of vascular endothelial growth factor and increasing the production of the angiogenesis inhibitor thrombospondin-1 [3]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 890.2±65.0 °C at 760 mmHg |
| Molecular Formula | C32H34Cl2N4O4S |
| Molecular Weight | 641.608 |
| Flash Point | 492.2±34.3 °C |
| Exact Mass | 640.167786 |
| PSA | 110.96000 |
| LogP | 4.00 |
| Appearance | light yellow to light brown |
| Vapour Pressure | 0.0±0.3 mmHg at 25°C |
| Index of Refraction | 1.656 |
| Storage condition | Store at +4°C |
| Water Solubility | DMSO: ≥20mg/mL |
|
~% 
477575-56-7 |
| Literature: US2003/125370 A1, ; |
| Precursor 1 | |
|---|---|
| DownStream 0 | |