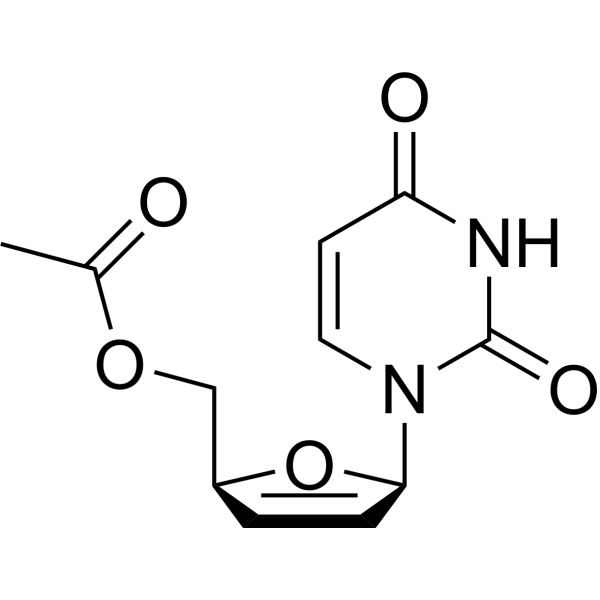
15379-29-0
| Name | 5-fluoro-1-[(2R,5S)-5-(hydroxymethyl)-2,5-dihydrofuran-2-yl]pyrimidine-2,4-dione |
|---|---|
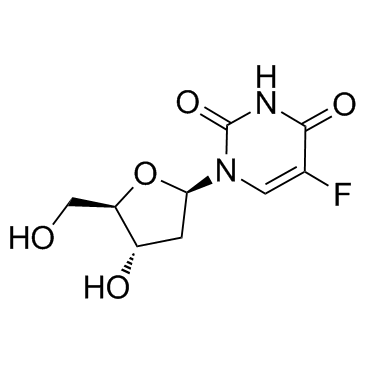
| Synonyms | 2',3'-Didehydro-2',3'-dideoxy-hexopyranosyl cytidine |
| Description | 2′,3′-Didehydro-2′,3′-dideoxy-5-fluorouridine is a purine nucleoside analog. Purine nucleoside analogs have broad antitumor activity targeting indolent lymphoid malignancies. Anticancer mechanisms in this process rely on inhibition of DNA synthesis, induction of apoptosis, etc[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.57g/cm3 |
|---|---|
| Molecular Formula | C9H9FN2O4 |
| Molecular Weight | 228.18 |
| Exact Mass | 228.05500 |
| PSA | 84.32000 |
| Index of Refraction | 1.609 |
|
~75% 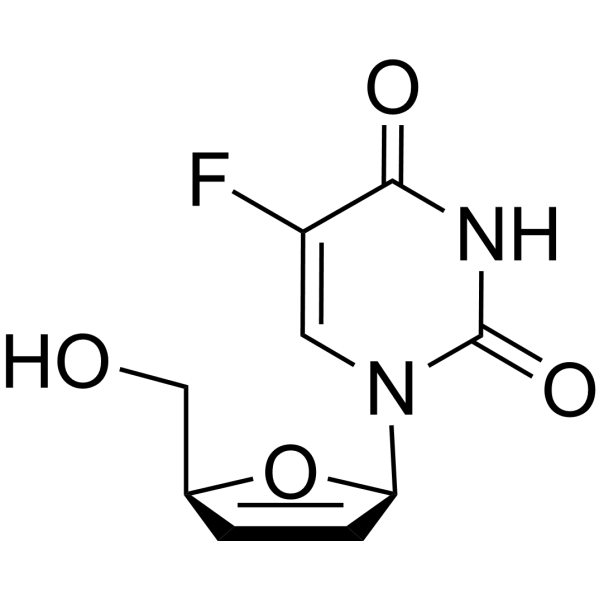
15379-29-0 |
| Literature: Joshi, Bhalchandra V.; Rao, T. Sudhakar; Reese, Colin B. Journal of the Chemical Society, Perkin Transactions 1: Organic and Bio-Organic Chemistry (1972-1999), 1992 , # 19 p. 2537 - 2544 |
|
~% 
15379-29-0 |
| Literature: Coe, Paul L.; Talekar, Ratnakar R.; Walker, Richard T. Journal of Fluorine Chemistry, 1994 , vol. 69, # 1 p. 19 - 24 |
|
~% 
15379-29-0 |
| Literature: Joshi, Bhalchandra V.; Rao, T. Sudhakar; Reese, Colin B. Journal of the Chemical Society, Perkin Transactions 1: Organic and Bio-Organic Chemistry (1972-1999), 1992 , # 19 p. 2537 - 2544 |
| Precursor 3 | |
|---|---|
| DownStream 0 | |