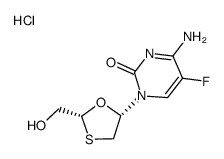
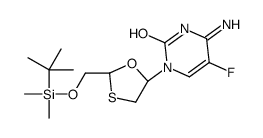
143491-57-0
| Name | Emtricitabine |
|---|---|
| Synonyms |
Emtricitabine
EMTRICITABIN Emtriva 4-Amino-5-fluor-1-[(2R,5S)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl]pyrimidin-2(1H)-on EMTRITABINE 4-amino-5-fluoro-1-[(2R,5S)-2-(hydroxyméthyl)-1,3-oxathiolan-5-yl]pyrimidin-2(1H)-one Coviracil 4-Amino-5-fluoro-1-((2R,5S)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl)pyrimidin-2(1H)-one FTC (-)-β-L-FTC Entricitabine (-)-FTC 4-Amino-5-fluoro-1-[(2R,5S)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl]-2(1H)-pyrimidinone SM-Q MFCD00870151 4-Amino-5-fluoro-1-[(2R,5S)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl]pyrimidin-2(1H)-one 2'-deoxy-5-fluoro-3'-thiacytidine UNII-ULS8902U4O |
| Description | Emtricitabine is a nucleoside reverse transcriptase inhibitor (NRTI) with an EC50 of 0.01 µM in PBMC cell. It is an antiviral drug for the treatment of HIV infection. |
|---|---|
| Related Catalog | |
| Target |
EC50: 0.01 µM (NRTI, PBMC), 0.026 µM (NRTI, HeLa cell)[1] |
| In Vitro | Emtricitabine has in vitro activity against both laboratory strains of HIV-1 and HIV-2 and clinical isolates of HIV-1. The 50% effective concentration (EC50) ranges from 0.002 to 1.5 µ mol/L, depending on the viral isolate and cell line used. Emtricitabine demonstrates in vitro synergy with zidovudine and stavudine and additive in vitro activity when combines with zalcitabine or didanosine[1]. |
| In Vivo | Reproductive and developmental toxicology studies are conducted with emtricitabine. Oral doses up to 1000 mg/kg/day provided daily area under the curve (AUC0→24) exposure to pregnant animals approximately 60- (mice) to 120-fold (rabbits) higher than that in humans at the recommended dose of 200 mg given once per day. In a mouse fertility study, emtricitabine had no effect on fertility, sperm count, or early embryonic development. There is no increased incidence of malformations in mouse and rabbit embryofetal toxicology studies. The development and fertility of F1 progeny are unaffected by emtricitabine in a mouse pre- and post-natal study. These data demonstrate a favorable pre-clinical reproductive safety profile for emtricitabine[2]. |
| Cell Assay | EA.hy926 cells were plated in a 12-, 24- or 96-well plates and grown in DMEM media supplemented with 3% FCS. Endothelial cells from PARP+/+and PARP-/- mice were isolated and cultured. Cell viability was determined by the reduction of yellow MTT into a purple formazan product by mitochondrial dehydrogenases of metabolically active cells. Following the treatment period, the experimental medium was removed and 100 μL MTT (1 mg/mL) added. After 1 h incubation, the MTT solution was carefully removed and the purple crystals were solubilized in 100 μL of DMSO. The DMSO was transferred to an ELISA plate and absorbance measured at 550 nm with a 620 nm[3]. |
| Animal Admin | Mice: Emtricitabine (free base) is suspended in 0.5% aqueous methylcellulose and given by gavage, with the daily dose divided into two equal installments administered approximately 6 h apart. The dose volume is 5 mL/kg/dose (10 mL/kg/day). In 1- and 6-month oral toxicity studies in mice, the maximum tolerated dose of emtricitabine is >3000 mg/kg/day. However, dose-range-finding studies are performed in pregnant CD-1 mice and in New Zealand White rabbits at top doses of 1000 mg/kg/day[2]. Rabbits: Mature artificially inseminated rabbits are given emtricitabine on gestational day 7 through 19. On gestational day 19, blood samples for toxicokinetics are taken from five satellite does in each group at 30–60 min prior to dosing, and at 1, 3, 7, and 12 h after the first daily-dose (prior to the second daily-dose). On gestational day 20, the satellite does are sacrificed at 1 h after the final dose, and maternal blood and fetal umbilical blood samples are collected for toxicokinetics[2]. |
| References |
| Density | 1.8±0.1 g/cm3 |
|---|---|
| Boiling Point | 443.3±55.0 °C at 760 mmHg |
| Melting Point | 136-140°C |
| Molecular Formula | C8H10FN3O3S |
| Molecular Weight | 247.247 |
| Flash Point | 221.9±31.5 °C |
| Exact Mass | 247.042694 |
| PSA | 115.67000 |
| LogP | -0.41 |
| Vapour Pressure | 0.0±2.4 mmHg at 25°C |
| Index of Refraction | 1.731 |
| Storage condition | -20°C Freezer |
| Hazard Codes | Xi |
|---|---|
| Safety Phrases | S26-S36 |
| RIDADR | 2446 |
| WGK Germany | 3 |
| Packaging Group | III |
| Hazard Class | 6.1 |
| HS Code | 2934999090 |
|
~79% 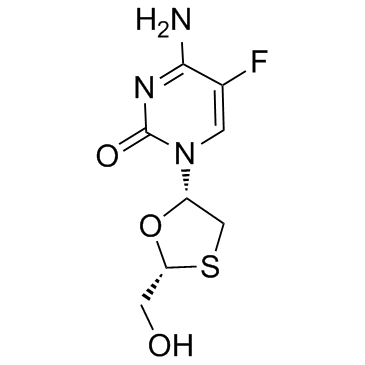
143491-57-0 |
| Literature: MATRIX LABORATORIES LIMITED Patent: WO2009/84033 A2, 2009 ; Location in patent: Page/Page column 10 ; |
|
~63% 
143491-57-0 |
| Literature: Esteve Química, S.A. Patent: EP2377862 A1, 2011 ; Location in patent: Page/Page column 7 ; |
|
~% 
143491-57-0 |
| Literature: WO2011/83484 A2, ; Page/Page column 17 ; |
|
~% 
143491-57-0 |
| Literature: WO2011/95987 A1, ; Page/Page column 39 ; |
|
~% 
143491-57-0 |
| Literature: Journal of Medicinal Chemistry, , vol. 36, # 2 p. 181 - 195 |
|
~% 
143491-57-0 |
| Literature: WO2011/95987 A1, ; |
| Precursor 3 | |
|---|---|
| DownStream 1 | |
| HS Code | 2934999090 |
|---|---|
| Summary | 2934999090. other heterocyclic compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |