5168-36-5
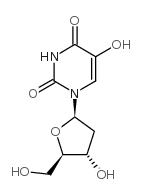
| Name | 5-hydroxy-2'-deoxyuridine |
|---|---|
| Synonyms |
Uridine,2'-deoxy-5-hydroxy
2'-Deoxy-5-hydroxyuridine Oh-5-du |
| Description | 5-Hydroxy-2'-deoxyuridine (5-OHdU) is a major stable oxidation product of 2'-Deoxycytidine. 5-Hydroxy-2'-deoxyuridine can be incorporated into DNA in vitro by DNA polymerase[1]. |
|---|---|
| Related Catalog | |
| Target |
Human Endogenous Metabolite |
| In Vitro | To study the specificity of nucleotide incorporation opposite 5-hydroxypyrimidines in template DNA, 18- and 45-member oligodeoxyribonucleotides, containing an internal 2'-deoxy-5-hydroxyuridine (5-OHdU) in two different sequence contexts, were used. Translesion synthesis past 2'-deoxy-5-hydroxyuridine (5-OHdU) in both oligonucleotides occurred, but pauses both opposite, and one nucleotide prior to, the modified base in the template is observed. The specificity of nucleotide incorporation opposite 2'-deoxy-5-hydroxyuridine (5-OHdU) in the template is sequence context dependent. In one sequence context, dA is the principal nucleotide incorporated opposite 2'-deoxy-5-hydroxyuridine (5-OHdU). However in a second sequence context, dC is the predominant base incorporated opposite 2'-deoxy-5-hydroxyuridine (5-OHdU). In that same sequence context, dC is also the predominant nucleotide incorporated opposite 2'-deoxy-5-hydroxyuridine (5-OHdU)[1]. |
| References |
| Density | 1.698g/cm3 |
|---|---|
| Molecular Formula | C9H12N2O6 |
| Molecular Weight | 244.20100 |
| Exact Mass | 244.07000 |
| PSA | 124.78000 |
| Index of Refraction | 1.654 |
| Storage condition | 2-8°C |
| Precursor 0 | |
|---|---|
| DownStream 3 | |