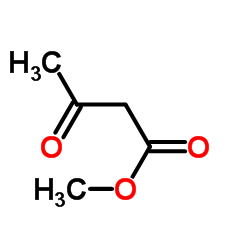
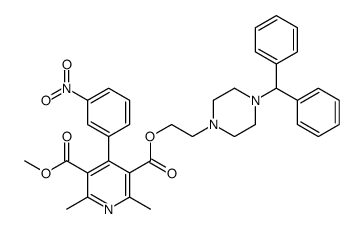
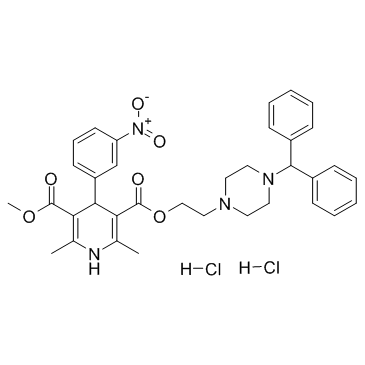
89226-75-5
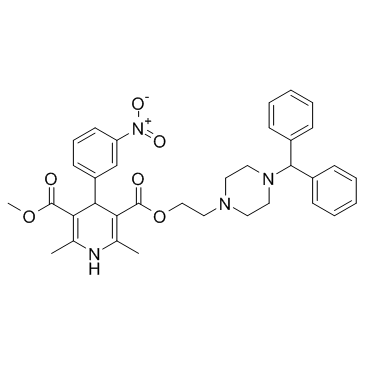
| Name | Manidipine Dihydrochloride |
|---|---|
| Synonyms |
3,5-Pyridinedicarboxylic acid, 1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)-, 2-[4-(diphenylmethyl)-1-piperazinyl]ethyl methyl ester, hydrochloride (1:1)
Manidipine hydrochloride 2-[4-(Diphenylmethyl)piperazin-1-yl]ethyl methyl 2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate hydrochloride (1:1) MFCD00896434 2-[4-(Diphenylmethyl)-1-piperazinyl]ethyl methyl 2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydro-3,5-pyridinedicarboxylate hydrochloride (1:1) Manidipine 2HCl Manidipine (dihydrochloride) |
| Description | Manidipine 2Hcl (CV-4093) is a dihydropyridine compound and a calcium channel blocker for Ca2+ current with IC50 of 2.6 nM. IC50 value: 2.6 nMTarget: calcium channelManidipine is described to block T-type Ca2+ channels specifically and is also described to have a high selectivity for the vasculature, presenting negligible cardiodepression as compared to other Ca2+ channel antagonists. Manidipine is also described to not significantly affect norepinephrine levels, suggesting a lack of sympathetic activation with this compound. Manidipine reduces pro-inflammatory cytokines secretion in human endothelial cells and macrophages. Manidipine, unlike other third-generation dihydropyridine derived drugs, blocks T-type calcium channels present in the efferent glomerular arterioles, reducing intraglomerular pressure and microalbuminuria. |
|---|---|
| Related Catalog | |
| References |
[4]. Roca-Cusachs A, Triposkiadis F. Antihypertensive effect of manidipine. Drugs. 2005;65 Suppl 2:11-9. |
| Boiling Point | 722ºC at 760 mmHg |
|---|---|
| Melting Point | 211 °C(dec.) |
| Molecular Formula | C35H40Cl2N4O6 |
| Molecular Weight | 683.62 |
| PSA | 116.93000 |
| LogP | 6.48280 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Hazard Codes | Xi |
|---|---|
| HS Code | 2942000000 |
|
~% 
89226-75-5 |
| Literature: WO2011/23954 A2, ; Page/Page column 18 ; |
|
~% 
89226-75-5 |
| Literature: WO2011/23954 A2, ; |
|
~% 
89226-75-5 |
| Literature: Chemical and Pharmaceutical Bulletin, , vol. 33, # 9 p. 3787 - 3797 |
|
~% 
89226-75-5 |
| Literature: Chemical and Pharmaceutical Bulletin, , vol. 33, # 9 p. 3787 - 3797 |
|
~14% 
89226-75-5 |
| Literature: Meguro; Aizawa; Sohda; Kawamatsu; Nagaoka Chemical and Pharmaceutical Bulletin, 1985 , vol. 33, # 9 p. 3787 - 3797 |
|
~17% 
89226-75-5 |
| Literature: Meguro; Aizawa; Sohda; Kawamatsu; Nagaoka Chemical and Pharmaceutical Bulletin, 1985 , vol. 33, # 9 p. 3787 - 3797 |
| Precursor 7 | |
|---|---|
| DownStream 1 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |