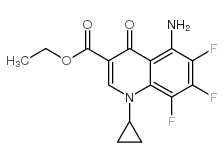
110871-86-8
| Name | sparfloxacin |
|---|---|
| Synonyms |
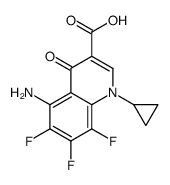
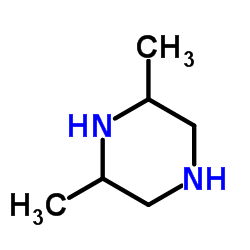
5-Amino-1-cyclopropyl-7-[(3R,5S)-3,5-dimethyl-1-piperazinyl]-6,8-difluoro-4-oxo-1,4-dihydro-3-quinolinecarboxylic acid
5-amino-1-cyclopropyl-7-(3,5-dimethyl-1-piperazinyl)-6,8-difluoro-1,4-dihydro-4-oxo-3-quinolinecarboxylic acid Sparcin PD 1315-1 Zagam at4140 5-amino-1-cyclopropyl-6,8-difluoro-7-(cis-3,5-dimethyl-1-piperazinyl)-1,4-dihydro-4-oxoquinoline-3-carboxylic acid SULFAMIC ACID SPARFLOXACIN (BASE AND/OR UNSPECIFIED SALTS) (cis)-5-amino-1-cyclopropyl-7-(3,5-dimethyl-1-piperazinyl)-6,8-difluoro-1,4-dihydro-4-oxo-3-quinolinecarboxylic acid Spara 5-amino-1-cyclopropyl-7-[(3R,5S)-3,5-dimethylpiperazin-1-yl]-6,8-difluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic acid ci978 5-amino-1-cyclopropyl-7-((3R,5S)-3,5-dimethylpiperazin-1-yl)-6,8-difluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic acid MFCD00869619 Sparfloxacin Sparfloxaci SPARFLOXACIN, ANTIBIOTIC FOR CULTURE MEDIA USE ONLY cis-5-amino-1-cyclopropyl-7-(3,5-dimethyl-1-piperazinyl)-6,8-difluoro-1,4-dihydro-4-oxo-3-quinoline carboxylic acid Parox |
| Description | Sparfloxacin is a fluoroquinolone antibiotic, shows broad and potent antibacterial activity.Target: AntibacterialSparfloxacin shows broad and potent antibacterial activity. Its MICs for 90% of the strains tested are 0.1 to 0.78 μg/ml against gram-positive organisms, such as members of the genera Staphylococcus , Streptococcus and Enterococcus , and 0.0125 to 1.56 μg/ml against gram-negative organisms, such as members of the family Enterobacteriaceae and the genera Pseudomona . Its MICs are 0.025 to 0.78 μg/ml against glucose nonfermenters, 0.2 to 0.78 μg/ml against anaerobes, 0.0125 to 0.05 μg/ml against Legionella. Sparfloxacin showed good oral efficacy against systemic infections with Staphylococcus aureus , Streptococcus pyogenes , Streptococcus pneumoniae , Escherichia coli , and Pseudomonas aeruginosa in mice [1]. Sparfloxacin targets DNA gyrase and inhibits DNA synthesis [2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 640.4±55.0 °C at 760 mmHg |
| Melting Point | 265°C |
| Molecular Formula | C19H22F2N4O3 |
| Molecular Weight | 392.400 |
| Flash Point | 341.1±31.5 °C |
| Exact Mass | 392.165985 |
| PSA | 100.59000 |
| LogP | 1.20 |
| Vapour Pressure | 0.0±2.0 mmHg at 25°C |
| Index of Refraction | 1.627 |
| Storage condition | Store at 0-5°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi:Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 2 |
| RTECS | VB1986500 |
|
~61% 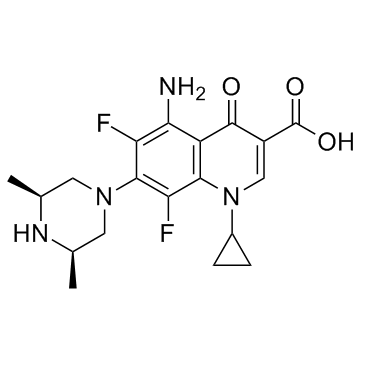
110871-86-8 |
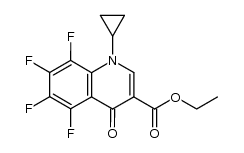
| Literature: Miyamoto, Teruyuki; Matsumoto, Jun-ichi; Chiba, Katsumi; Egawa, Hiroshi; Shibamori, Koh-ichiro; et al. Journal of Medicinal Chemistry, 1990 , vol. 33, # 6 p. 1645 - 1656 |
|
~% 
110871-86-8 |
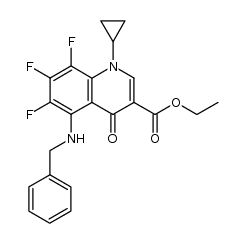
| Literature: Journal of Medicinal Chemistry, , vol. 33, # 6 p. 1645 - 1656 |
|
~% 
110871-86-8 |
| Literature: Journal of Medicinal Chemistry, , vol. 33, # 6 p. 1645 - 1656 |
|
~% 
110871-86-8 |
| Literature: Journal of Medicinal Chemistry, , vol. 33, # 6 p. 1645 - 1656 |
| Precursor 5 | |
|---|---|
| DownStream 0 | |