364-62-5
| Name | metoclopramide |
|---|---|
| Synonyms |
Pramin
Imperan MAXOLON Reglan Maxeran EINECS 206-662-9 Plasil metoclopramide Eucil Primperan metoclopramidum [INN_la] 4-Amino-5-chloro-N-[2-(diethylamino)ethyl]-2-methoxybenzenecarboximidic acid 4-Amino-5-chloro-N-[2-(diethylamino)ethyl]-2-methoxybenzamide,Methoxychloroprocainamide 4-Amino-5-chloro-N-[2-(diethylamino)ethyl]-2-methoxybenzamide |
| Description | Metoclopramide is a dopamine D2 antagonist that is used as an antiemetic.IC50 Value:Target: D2 ReceptorMetoclopramide is a dopamine receptor antagonist which has been used for treatment of a variety of gastrointestinal symptoms over the last thirty years. In various countries, metoclopramide is the antiemetic drug of choice in pregnant women. Findings provide reassurance regarding the safety of metoclopramide for the fetus when the drug is given to women to relieve nausea and vomiting during pregnancy. Evidence also supports its use for gastroparesis (poor stomach emptying) and gastroesophageal reflux disease. It appears to bind to dopamine D2 receptors where it is a receptor antagonist, and is also a mixed 5-HT3 receptor antagonist/ 5-HT4 receptor agonist. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 454.8±55.0 °C at 760 mmHg |
| Melting Point | 146-148°C |
| Molecular Formula | C14H22ClN3O2 |
| Molecular Weight | 299.80 |
| Flash Point | 228.9±31.5 °C |
| PSA | 67.59000 |
| LogP | 3.10 |
| Vapour Pressure | 0.0±1.2 mmHg at 25°C |
| Index of Refraction | 1.545 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302-H362 |
| Precautionary Statements | P263 |
| Hazard Codes | Xn |
| Risk Phrases | 22-64 |
| Safety Phrases | 36/37 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2924299090 |
|
~% 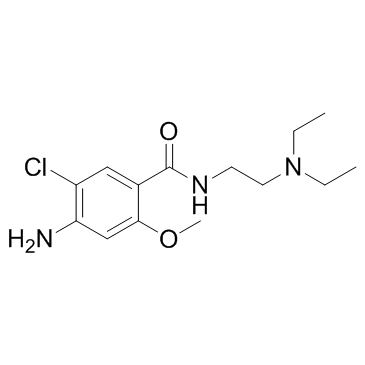
364-62-5 |
| Literature: US4242507 A1, ; |
|
~% 
364-62-5 |
| Literature: Journal of Medicinal Chemistry, , vol. 36, # 21 p. 3166 - 3170 |
|
~% 
364-62-5 |
| Literature: Zhichkin, Paul E.; Peterson, Lisa H.; Beer, Catherine M.; Rennells, W. Martin Journal of Organic Chemistry, 2008 , vol. 73, # 22 p. 8954 - 8959 |
|
~% 
364-62-5 |
| Literature: Hermange, Philippe; Lindhardt, Anders T.; Taaning, Rolf H.; Bjerglund, Klaus; Lupp, Daniel; Skrydstrup, Troels Journal of the American Chemical Society, 2011 , vol. 133, # 15 p. 6061 - 6071 |
|
~% 
364-62-5 |
| Literature: Archiv der Pharmazie, , vol. 313, # 4 p. 297 - 300 |
|
~% 
364-62-5 |
| Literature: Journal of the American Chemical Society, , vol. 133, # 15 p. 6061 - 6071 |
|
~% 
364-62-5 |
| Literature: Journal of Medicinal Chemistry, , vol. 36, # 21 p. 3166 - 3170 |
|
~% 
364-62-5 |
| Literature: Archiv der Pharmazie, , vol. 313, # 4 p. 297 - 300 |
|
~% 
364-62-5 |
| Literature: Archiv der Pharmazie, , vol. 313, # 4 p. 297 - 300 |
| Precursor 8 | |
|---|---|
| DownStream 6 | |
| HS Code | 2924299090 |
|---|---|
| Summary | 2924299090. other cyclic amides (including cyclic carbamates) and their derivatives; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |









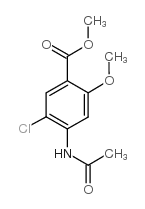
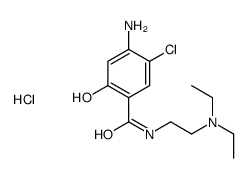
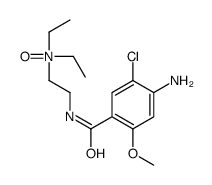

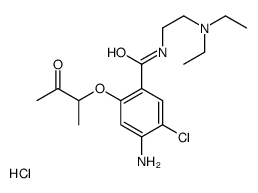
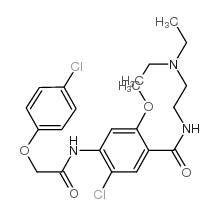
![4-amino-5-chloro-N-[2-(diethylamino)ethyl]-2-hydroxybenzamide structure](https://image.chemsrc.com/caspic/378/38339-95-6.png)