382180-17-8
| Name | N'-hydroxy-N-pyridin-3-yloctanediamide |
|---|---|
| Synonyms |
S2190_Selleck
N-Hydroxy-N'-3-pyridinyloctanediamide N-Hydroxy-N'-(pyridin-3-yl)octanediamide N1-Hydroxy-N8-3-pyridinyl-octanediamide N-Hydroxy-N'-(3-pyridinyl)octanediamide pyroxamide N-Hydroxy-N'-3-pyridinyl octane diamide |
| Description | Pyroxamide is a potent inhibitor of histone deacetylase 1 (HDAC1) with an ID50 of 100 nM. Pyroxamide can induce apoptosis and cell cycle arrest in leukemia. |
|---|---|
| Related Catalog | |
| Target |
ID50: 100 nM[1] |
| In Vitro | Pyroxamide (1.25-20.0 μM; 24-72 hours) suppresses RD and RH30B cells growth, pyroxamide resulted in 44% dead cells for 72 h at 20.0 μM, results in 86% dead cells in culture[1]. Pyroxamide (10.0-20.0 μM; 48 hours) shows sub-G1 fractions of 45.0% and 72.3% at 10.0 and 20.0 μM, respectively[1]. Cell Viability Assay[2] Cell Line: RD cells; RH30B cells Concentration: 1.25-20.0 μM Incubation Time: 24 hours; 48 hours; 72 hours Result: Resulted in a cell growth decrease in RD and RH30B cells. Cell Cycle Analysis[2] Cell Line: RD cells; RH30B cells Concentration: 10.0 μM; 20.0 μM Incubation Time: 48 hours Result: Increased the sub-G1 fractions at 48 hours compared with control samples. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Molecular Formula | C13H19N3O3 |
| Molecular Weight | 265.308 |
| Exact Mass | 265.142639 |
| PSA | 91.32000 |
| LogP | 0.04 |
| Appearance | white to beige |
| Index of Refraction | 1.570 |
| Storage condition | ?20°C |
| Water Solubility | DMSO: soluble10mg/mL (clear solution) |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 |
| Hazard Codes | Xn |
| Risk Phrases | 22 |
| RIDADR | NONH for all modes of transport |
| HS Code | 2933399090 |
|
~46% 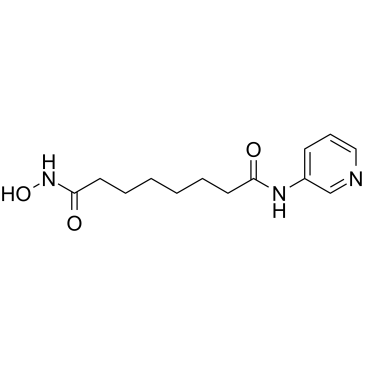
382180-17-8 |
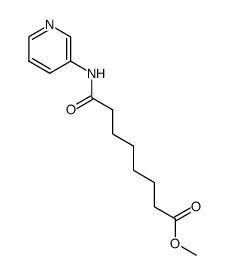
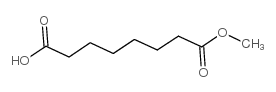
| Literature: Remiszewski, Stacy W.; Sambucetti, Lidia C.; Atadja, Peter; Bair, Kenneth W.; Cornell, Wendy D.; Green, Michael A.; Howell, Kobporn Lulu; Jung, Manfred; Kwon, Paul; Trogani, Nancy; Walker, Heather Journal of Medicinal Chemistry, 2002 , vol. 45, # 4 p. 753 - 757 |
|
~% 
382180-17-8 |
| Literature: Remiszewski, Stacy W.; Sambucetti, Lidia C.; Atadja, Peter; Bair, Kenneth W.; Cornell, Wendy D.; Green, Michael A.; Howell, Kobporn Lulu; Jung, Manfred; Kwon, Paul; Trogani, Nancy; Walker, Heather Journal of Medicinal Chemistry, 2002 , vol. 45, # 4 p. 753 - 757 |
| Precursor 2 | |
|---|---|
| DownStream 0 | |
| HS Code | 2933399090 |
|---|---|
| Summary | 2933399090. other compounds containing an unfused pyridine ring (whether or not hydrogenated) in the structure. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |